Legally Reviewed
This article has been reviewed, fact-checked, and updated by Matthew Dolman and Stanley Gipe. Matthew and Stanley have a combined 45 years of experience practicing law and have represented over 10,000 clients who sustained injuries as a result of negligence. Matthew and Stanley focus much of their practice on mass tort litigation handling claims similar to the Suboxone tooth decay lawsuit. Stanley Gipe is a member of the Plaintiff’s Steering Committee in the Suboxone Lawsuit. This content should not be taken as legal advice from an attorney.
Fact-Checked
Dolman Law Group and our attorneys do our best to ensure the information in this article is up-to-date and accurate. This article should not be taken as advice from an attorney. However, if you need specific legal advice about your case, contact us directly.
Chat With An Agent
Suboxone is a medication commonly prescribed to individuals with opioid addiction. The medicine works by providing a way to end addiction to opioids, curb opioid withdrawal symptoms, and reduce the effects and potency of opioids taken in relapse.
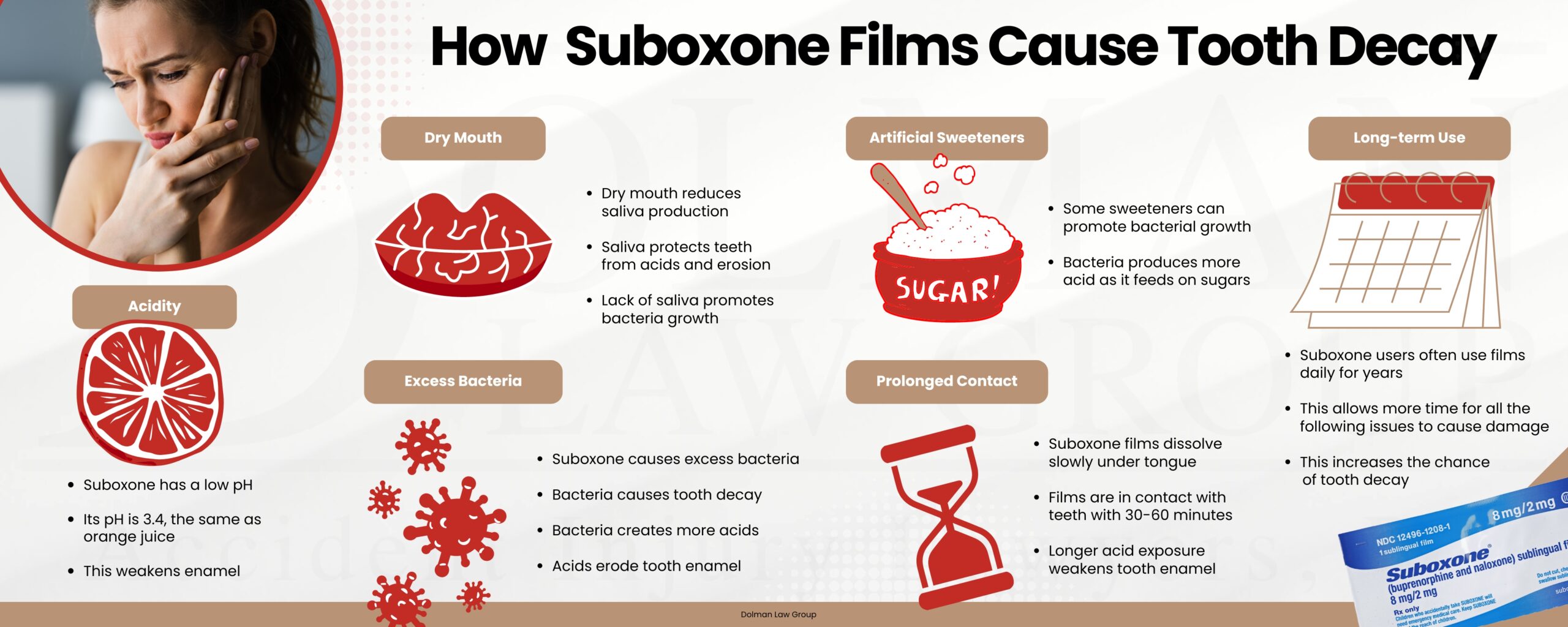
However, the sublingual film version of Suboxone has recently been shown to cause tooth decay and other dental injuries because it contains buprenorphine. The Suboxone sublingual film has proven to be acidic and degrades the protective outer layer of teeth and tooth enamel.
If you or a loved one have experienced dental problems while taking Suboxone before 2022, you may be able to recover compensation. The Suboxone tooth decay lawsuit is beginning to gain traction.
Dolman Law Group is here to help you understand your rights and take the necessary steps to get the money you need to pay your medical bills and rebuild your life.
If you have experienced tooth decay or other dental issues after using the oral film version of Suboxone, contact us online or by calling 727-451-6900.
Table of Contents
- Recent Rise in Suboxone Tooth Decay Lawsuits
- Updates on the Suboxone Lawsuit - December 2025
- Can I Sue Suboxone for Tooth Decay?
- What is Suboxone?
- Suboxone and Tooth Decay
- What Evidence Is There That Suboxone Causes Tooth Decay?
- FDAs Response to Suboxone Tooth Decay
- Damages in a Suboxone Lawsuit
- How Dolman Law Group Can Lead Your Tooth Decay Suboxone Case
- Learn Whether You Qualify for a Suboxone Dental Lawsuit
Recent Rise in Suboxone Tooth Decay Lawsuits

There has recently been a significant increase in Suboxone lawsuits. In November 2023, 14 new product liability lawsuits were filed in federal courts against Indivior, the maker of Suboxone.
This brings the total number of Suboxone tooth decay lawsuits filed to over 100, while the nationwide multi-district litigation has consolidated at least 44 cases as of April 2024. This number is only expected to rise as the hundreds of thousands of people who took Suboxone and are suffering from severe dental problems, including tooth loss, come forward.
The rise in Suboxone tooth decay lawsuits is likely due to several factors. But the most common reasons are likely:
- Increased awareness that Suboxone can cause tooth decay as the word spreads
- The popularity of Suboxone to treat opioid addiction is increasing
- More people are starting to experience tooth decay and are finding out about the link when they visit their dentist for dental health issues.
- Pharmaceutical companies (such as Invidior PLC, the manufacturer of Suboxone) responsible for the opioid crisis have created products designed to overcome opioid dependence, which include Suboxone. Opioid addiction treatment has become a cottage industry itself. Suboxone is considered best in class for the treatment of Opioid use.
Updates on the Suboxone Lawsuit – December 2025
We aim to provide the most up-to-date information on Suboxone tooth decay lawsuits online. The Dolman Law Group (through our sister firm, Dolman Russo) is representing individuals who have suffered severe tooth decay, tooth loss, and other dental injuries related to using Suboxone sublingual strips. What began as the Suboxone class action lawsuit has evolved into multidistrict litigation.
In February, the Judicial Panel on Multidistrict Litigation (JPML) heard arguments from plaintiff and defense lawyers. Ultimately, the JPML consolidated all federal Suboxone tooth decay lawsuits before Judge Philip Calabrese in the United States District Court for the Northern District of Ohio.
We believe the scientific evidence in the Suboxone tooth decay litigation to be powerful. The vast number of adverse dental outcomes reported to the U.S. Food and Drug Administration (FDA) provides information on how the acidic nature of sublingual Suboxone destroys tooth enamel.
All Suboxone lawsuits to date allege that Indivior designed the sublingual medication to be acidic. Further, the lawsuits allege that Indivior knew and failed to warn consumers that Suboxone film causes severe dental injuries.
Here are some of the latest developments in the Suboxone tooth decay litigation:
Earlier this month, the number of Suboxone lawsuits filed in federal court dipped slightly to 1,871 active cases. While some dismissals were expected due to missing paperwork or deadlines, new cases are still being filed, and many existing claims remain on track for trial.
One encouraging sign came directly from the court. In a recent ruling, the federal judge overseeing the Suboxone MDL allowed plaintiff Michelle Robinson to keep her case active, despite having missed an earlier deadline. The court acknowledged that she was incarcerated for much of 2025 and was unable to submit her documents on time. After her release, she followed through, and the court agreed she had a valid reason for the delay.
At Dolman Law Group, we understand that life doesn’t always go as planned. That’s why we’re committed to helping people who have been hurt by Suboxone, no matter where they are in their recovery journey. Attorney Stan Gipe, our lead trial lawyer, is actively involved in this case as a member of the Plaintiffs’ Steering Committee—the legal team leading the fight on behalf of everyone affected by Suboxone’s dangerous side effects.
If you’ve suffered dental damage from Suboxone film, you may have legal options. Reach out to Dolman Law Group to learn how we can help. Your consultation is always free, and we’re here to answer your questions with care and compassion.
Thousands of people who trusted Suboxone as part of their recovery now face a second battle—trying to afford the dental care they need after suffering serious tooth decay, erosion, and tooth loss. And while the road to justice hasn’t been fast, things are finally accelerating in court.
In October 2025, the judge overseeing the Suboxone tooth damage litigation issued several new case management orders aimed at pushing the process forward. These include:
- Mandatory census forms within 60 days for all new filings after October 1, 2025
- A June 1, 2026, deadline for older cases to submit missing paperwork
- A court order requiring Indivior and Aquestive to turn over key internal records, including FDA filings and reports of nearly 90 dental injury complaints
These updates are helping ensure that strong cases move forward and that the companies responsible are held accountable.
Dolman Law Group Is Helping Lead the Fight
We’re proud to share that Attorney Stan Gipe, Dolman Law Group’s lead trial lawyer, has been appointed to the Plaintiffs’ Steering Committee in the Suboxone MDL. That means the same attorney representing our clients is helping lead the national litigation strategy.
Stan’s role includes overseeing key discovery issues, helping select bellwether trial cases, and making sure the voices of people hurt by Suboxone are heard in court.
What You Can Do Now
If Suboxone caused your dental damage, don’t wait. With over 11,000 lawsuits already filed and more on the way, these new court procedures are designed to separate the strongest claims from those that may not qualify.
If you’ve been harmed by Suboxone, contact Dolman Law Group today. We’ll help you understand your rights, gather the documents needed for your case, and make sure your claim is positioned for success—whether through settlement or trial.
The Court Is Taking Delays Seriously
At a recent hearing, the judge overseeing the federal Suboxone litigation made it clear: delays will no longer be tolerated. Some outside treatment providers have still not turned over the medical records needed to support patients’ claims. The court is now threatening serious penalties, known as contempt sanctions, for those who continue to ignore court orders.
On the patient side, a small number of lawsuits were dismissed because those individuals didn’t complete the paperwork required by the court. While that may sound alarming, this move actually helps serious plaintiffs by clearing out unsupported claims so the court can focus on real, documented injuries like yours.
What You Can Do to Protect Your Claim
If you’ve filed a claim—or are thinking about it—it’s critical to submit all requested medical forms, dental records, and treatment authorizations. These documents show the timeline of your Suboxone use and the damage you’ve endured. The next round of plaintiff questionnaires (called Census Round 2) is about to begin, and meeting deadlines is more important than ever.
The Number of Cases Is Growing Fast
While the court docket officially lists 1,882 filings, legal experts believe over 20,000 patients have joined the litigation so far. Many cases are grouped into “batch filings” that include up to 100 people per complaint. These numbers show how widespread Suboxone’s damage is and how many patients are in the same situation you are.
We are Still Waiting on Settlement Discussions
There’s been a lot of speculation about when the Suboxone lawsuits will settle. While some hoped for an early resolution, the more likely timeline points to 2026, after key test trials get closer. These bellwether trials will be the first chance for juries to hear what Suboxone has done to patients, and that’s when real settlement talks are expected to begin.
At Dolman Law Group, we understand how hard it is to live with sudden, painful dental injuries, and how frustrating it is to wait for justice. If you’ve been harmed by Suboxone, contact our team for a free consultation. We’re here to guide you through the process and fight for the compensation you deserve.
What’s Holding Up the Lawsuits?
One of the biggest challenges in the Suboxone litigation right now is getting pharmacy and medical provider records. These documents are essential to prove that a plaintiff was prescribed Suboxone before their dental injuries began. Unfortunately, many providers have been dragging their feet, ignoring court orders, and failing to respond to repeated requests.
On August 26, 2025, the judge overseeing the Suboxone cases held a hearing to address four providers who still haven’t turned over records, despite months of follow-up. The providers didn’t show up in court, and the judge is now considering sanctions and legal fees. This is a strong sign that the court is serious about pushing these cases forward and giving the plaintiffs the ability to prove their cases.
More Victims Are Stepping Forward in Groups
We’re also seeing more lawsuits being filed in large groups, which helps reduce delays and costs. In one recent filing, 54 people from across the country joined together to sue the makers of Suboxone film. Each of them took the medication as prescribed and now suffers from severe tooth decay, gum erosion, and dental loss, injuries they say came with no warning.
They’re not alone. Many people took Suboxone as part of a treatment plan for addiction or chronic pain. They trusted the medication and their doctors. What they didn’t expect was to lose their teeth, their confidence, and their health.
Why This Matters to You
The judge is clearly growing impatient with the delays. A separate court order earlier this month required nearly 20 pharmacies to appear in court to explain why they’re withholding prescription records. These developments are essential to moving forward with trials and fair settlement discussions.
If you’ve experienced dental problems after taking Suboxone, it’s important to speak with an attorney who understands this process and can help you gather the documentation needed to support your case. At Dolman Law Group, we’re here to listen, explain your options, and fight for the justice you deserve.
You took the right steps and sought treatment. Let us help you take the next step toward accountability and justice. Reach out today to learn how we can help.
Recent Progress in the Suboxone Lawsuit
In July, the federal judge overseeing the Suboxone tooth decay lawsuits held a two-hour court hearing to check how things were moving. A lot was covered, and important agreements were reached, involving:
- Finalizing how missing documents will be handled
- Updating records and authorization forms
- Planning for how new claims filed after October 7, 2024, will be tracked
- Resolving disputes over how corporate representatives will be deposed
Two short deadlines were imposed on the parties:
- August 15, 2025 – Submit a plan for gathering more records
- September 29, 2025 – File summary fact sheets about claims and defenses
The next live court hearing is scheduled for September 9, 2025.
New Rules for Depositions
A separate court order now lays out how depositions (recorded interviews with witnesses) will be handled. Most will be in person unless both sides agree otherwise. For remote depositions, strict security and confidentiality rules are in place. If the plaintiffs want to speak with current or former employees of the drugmaker, the company must respond with two available dates within 10 days.
What Indivior May Not Understand
Indivior, the company behind Suboxone, may be underestimating the power of the injured users’ stories.
They seem to believe juries won’t care about the plaintiffs because they are in recovery. But jurors are people who will listen to how you worked hard to rebuild your life, followed your treatment plan, and were never told that the medication you trusted could cause irreversible dental damage.
Many of our clients describe:
- Cracked or missing teeth
- Full dentures at a young age
- Constant dental pain and massive out-of-pocket costs
- Embarrassment, anxiety, and lost self-esteem
These are not small injuries—they are life-changing. And the people behind these claims are not just patients—they are parents, caregivers, employees, and survivors.
Your Case Deserves to Be Heard
The Suboxone lawsuits are gaining momentum. More stories are coming to light. The legal system is moving toward trials, and your experience could be part of the change. Reach out to Dolman Law Group to learn more.
June 2025 brought some clarity to the legal battle. Here’s where things stand:
Let’s cut to the chase: there’s no widespread Suboxone settlement yet. Indivior, the drug’s manufacturer, has been playing a ruthless waiting game. They’ve skillfully avoided serious settlement talks, knowing that early negotiations would trigger a tsunami of new lawsuits. By holding off until closer to the statute of limitations deadline, Indivior can limit the total number of claims and significantly contain their financial exposure. It’s a calculated move to control the narrative and the cost.
Right now, the heavy lifting is all about the bellwether trials. These “test” cases are crucial in any mass tort, and the Suboxone MDL is no different. Both sides will use these trials to gauge the strength of their arguments, see how juries react, and get a better handle on what these claims are actually worth. The court has a detailed plan for selecting these cases, and the first trials are currently slated for 2026.
The discovery phase is still grinding away, with plaintiffs actively collecting medical records and other vital evidence. But here’s where it gets messy: getting pharmacy records for your Suboxone use has been a massive headache. Pharmacies aren’t exactly making it easy. Just last month (May 2025), the judge had to crack down, ordering giants like Walgreens to explain why they weren’t turning over required records. While some have finally complied, Walgreens is still dragging its feet and hasn’t offered a sufficient excuse.
As of July 1, 2025, the Suboxone MDL has about 900 active federal cases. But don’t let that number fool you. Many, many more are expected to be filed in bulk, thanks to a court order allowing attorneys to file up to 100 plaintiffs per complaint. This isn’t just about efficiency; it means attorneys can take their time vetting cases, leading to fewer non-viable claims flooding the MDL down the line. We’ll see the real surge in numbers as these bulk filings get processed.
June 2025 was a critical deadline for many plaintiffs, especially those in states with a three-year statute of limitations. This lines up with the June 2022 warning label update, when Indivior finally acknowledged the dental risks. Many people only became aware of the link after this, making June 2025 a crucial cutoff. This deadline undoubtedly triggered a surge of new filings, though the full impact, particularly from those bulk filings, will become clearer in the coming months.
So, what’s the bottom line for a settlement? Most legal experts believe that serious settlement talks won’t really heat up until after the initial bellwether trials play out in 2026. It just doesn’t make sense for Indivior or the plaintiffs to negotiate a global deal before they see how these test cases fare in court.
In essence, June 2025 marked a significant leap forward in the legal process and likely a huge increase in case filings. However, the path to a widespread settlement is still directly tied to those bellwether trials, which are still several months away.
In my opinion, don’t expect any talk of a global settlement in the Suboxone Tooth Decay MDL until 2026. And don’t take this as discouragement at all. Just because settlement negotiation talks have not begun doesn’t mean anything. Both sides are simply waiting on the outcome of the Bellweather trials before they make any costly moves.
If you’re living with painful dental problems after using Suboxone, you’re not alone—and you’re not without options. The Suboxone tooth decay litigation continues to move forward in federal court, with important updates unfolding that could impact your rights and your future.
Getting Records Shouldn’t Be This Hard—But It Is
One of the most frustrating challenges many patients face right now is accessing pharmacy records to prove they were prescribed Suboxone. Unfortunately, some large pharmacy chains haven’t made that process easy. Recently, the court had to step in, issuing a formal order asking several providers, including Walgreens, to explain delays in turning over these essential documents.
While some pharmacies have since complied, Walgreens still faces a hearing for its failure to meet the court’s timeline. For people wanting to move their case forward, this lack of cooperation feels like yet another setback. Hopefully, these issues will be resolved soon.
Trial Preparation Continues Despite Setbacks
At the same time, progress is being made in selecting which Suboxone cases could be among the first to go to trial. Out of the initial group of 500 plaintiffs, 48 people were removed, either because they withdrew from the lawsuit or couldn’t provide the necessary documentation. They’ve now been replaced with new plaintiffs, keeping the process moving.
This group is important because it’s where the court will eventually choose a small number of cases, known as bellwether trials, to help shape how future claims are handled. Even though the process may seem slow, every step forward matters.
Settlement Hopes: Realistic Timelines Matter
Understandably, many people are wondering when settlement talks might begin. Real settlement discussions are unlikely to start until late 2025 or beyond.
Why the wait? The drugmaker, Indivior, is likely holding off until the statute of limitations for filing lawsuits starts to close. After the statutory deadline, no new cases can be filed. It’s a strategic defense move that makes the wait even harder for those already dealing with long-term pain and expenses.
If you used Suboxone film to stay on track in your recovery and later began suffering from severe tooth decay, broken teeth, or painful dental problems, you are not alone, and you may have legal options.
The truth is finally coming to light. A growing number of lawsuits—nearly 900 and counting—claim that Indivior, the maker of Suboxone, failed to warn patients about the serious dental risks tied to long-term use of the film version of the drug.
Preparing for the First Trials
Federal courts are preparing for the first trials, and stories like yours could help shape the outcome. A new court order has launched the process for choosing the first cases to go before a jury. These early trials, called bellwether cases, will help show the human cost of this issue and how jurors are likely to respond.
A new court order has laid out the path to the first Suboxone trials. It begins with a review of 500 cases to collect and examine medical records. From that group, 100 cases will move forward into a deeper discovery phase, where more detailed information will be exchanged. After this stage, 15 cases will be chosen for trial consideration. Finally, four individual cases will be selected to proceed as the first bellwether trials.
When You Have Questions, Dolman Law Group Has the Answers
At Dolman Law Group, we believe every patient deserves answers and justice. If you’ve suffered dental injuries after using Suboxone film, our attorneys are here to listen, explain your options, and fight for your rights. We understand the courage it takes to speak up, and we’ll be with you every step of the way.
Contact us today for a free, confidential consultation. Your story matters. Let us help you tell it.
A federal multidistrict litigation (MDL) is underway in the U.S., involving hundreds of lawsuits that allege Suboxone (buprenorphine/naloxone) causes severe dental problems, including tooth decay and tooth loss. As of May 1, 2025, about 896 individual Suboxone dental injury cases are consolidated in MDL No. 3092 before Judge J. Philip Calabrese in the Northern District of Ohio. No bellwether trials have been held yet, and no settlements have been officially approved, but the pre-trial proceedings are ongoing.
Recent MDL developments
The court issued Case Management Order No. 14 to streamline new filings. It allows up to 100 plaintiffs to join in one complaint (with a single filing fee) in the MDL, helping to handle the influx of cases efficiently. By mid-April 2025, the parties had identified a pool of 500 plaintiffs for focused discovery, such as collecting dental records, as a step toward selecting bellwether trial candidates. Judge Calabrese has been holding regular status conferences (one was on April 17, 2025) to resolve discovery issues and keep the case on track for eventual trials.
Discovery is progressing: plaintiffs’ attorneys have signaled plans to take Rule 30(b)(6) corporate depositions of Indivior (Suboxone’s manufacturer) on topics such as how the company handled Suboxone dental complaints, details of the 2022 FDA-required label change (which added a dental warning), and the corporate structure of Indivior and its affiliates related to Suboxone’s development.
The court is also enforcing third-party compliance with subpoenas—for example, at the April status conference, the judge set a show-cause hearing for CVS, a pharmacy chain, over its failure to produce patient pharmacy records needed in the litigation. Overall, the MDL is moving through discovery, and no trial dates have been set yet, but the groundwork for bellwether trials later in 2025 is being laid.
Volume and Coordination
The last 30 days confirm that the Suboxone dental injury litigation is expanding rapidly and becoming more organized. The MDL’s innovative filing and case management orders are streamlining what could have been a chaotic influx of thousands of individual suits. For us, this means it’s now easier to add new cases to our files (lower fees and direct MDL filing). Still, it is also vital to diligently follow MDL procedures, ensuring that each client’s facts are correctly documented in joint complaints, etc.
We are mindful of the upcoming June 2025 cutoff for filings – Judge Calabrese explicitly warned that without a tolling deal, every case must be filed to preserve claims. Practically, we are racing against the clock this spring to sign up clients and bundle their filings before any statute runs. The court’s willingness to accommodate bulk filings is a boon, but it also expects compliance and organization. We see a trend of close judicial supervision: monthly status conferences, detailed orders on evidence gathering, and an explicit schedule for moving cases forward.
Bellwether Strategy
Both plaintiffs and defendants are now gearing up for the bellwether trial phase. The selection of 500 candidate cases and the narrowing process is essentially a vetting of the strongest examples on each side. Plaintiffs’ firms should strategize: which of their cases might be picked, and how to best position them (complete medical records, expert evaluations of dental damage, etc.).
Defense counsel, on the other hand, will analyze which claims seem most defensible or most severe. In the next few months, we may see jockeying over which specific cases are included in the bellwether pool. The March 14 orders indicate that Judge Calabrese wants a fair cross-section of cases—likely including a mix of demographics, durations of Suboxone use, and extent of dental injury—rather than just hand-picked extremes.
The implication is to prepare every case as if it could be that first trial. Being selected as a bellwether can spotlight a firm’s client, for better or worse, so readiness is key. The first bellwether trials, expected in late 2025, will heavily influence settlement values for all other cases. A significant plaintiff verdict could pressure Indivior to settle en masse, whereas a defense win might curb the momentum. Thus, the next 6-8 months are critical: it’s when evidence is gathered and test cases are chosen, effectively setting the stage for global settlement talks or protracted trial battles.
Defendant’s Posture
Indivior’s strategy in recent weeks appears to be resisting broad concessions and testing plaintiffs’ resolve. They opposed a blanket tolling agreement (which would have led to a flood of filings) and had earlier tried to knock out claims via preemption, but the court largely denied this. Internally, Indivior is likely assessing its exposure: with about 10,000+ lawsuits already filed or bundled, the potential liability could be significant. One notable factor is the profile of the plaintiffs – many are recovering opioid addicts treated with Suboxone.
The defense may hope that juries will be less sympathetic to such individuals or attribute their dental issues to poor personal health. However, we counter that these are highly sympathetic stories: people who got clean from opioids only to have Suboxone ruin their teeth. In the past 30 days, some commentary, including from the bench, hinted that underestimating these plaintiffs could be a costly mistake for the defense.
We may see Indivior pivot to a more conciliatory approach if the case counts continue to rise, for instance, engaging in mediation talks later in 2025 after a few bellwether cases are prepared. For now, however, no settlement overtures have been reported. Indivior defends each case, hires expert dentists to argue other causes of decay, and so on. Their liability insurance and financial statements, as noted in recent filings, acknowledge the MDL. Still, the company insists Suboxone is safe when used as directed (apart from the known risks now on the label).
The Suboxone multidistrict litigation (MDL 3092) is expected to gain significant momentum in the coming months. The June 2025 deadline marks the expiration of the statute of limitations (SOL) for failure-to-warn claims in states with two-year filing deadlines. Since the warning label for Suboxone sublingual films was updated in June 2022, any claims in those states must be filed by June of this year.
The Suboxone tooth decay lawsuits are consolidated in MDL No. 3092 in the U.S. District Court for the Northern District of Ohio. The litigation continues to expand rapidly, with 896 pending lawsuits as of early February 2025—an increase of 142 cases since January. This sharp rise suggests that more individuals are coming forward as awareness of Suboxone’s dental risks spreads.
Recent Individual Lawsuit Filings
New lawsuits continue to be filed across the country. Below are two notable recent cases:
- Suboxone Boston Case
A plaintiff in Boston, Massachusetts, alleges that prolonged Suboxone film use, even as prescribed, led to severe dental injuries. Despite no prior history of dental problems, the individual suffered:- Rapid tooth decay
- Multiple root canals
- Crowns and extractions
The lawsuit blames Suboxone’s acidic formulation, which dissolves under the tongue, for eroding tooth enamel and causing irreparable damage. This case underscores a recurring theme—patients followed prescription guidelines and maintained oral hygiene yet suffered serious harm due to insufficient warnings.
- Pennsylvania Suboxone Case
A Pennsylvania resident filed a lawsuit citing similar injuries. The plaintiff contends that Suboxone film use led to:- Severe and permanent dental damage
- Rampant cavities
- Cracked teeth
- Oral infections and tooth loss
The lawsuit attributes these injuries to Suboxone’s prolonged low-pH exposure in the mouth, which weakened enamel and promoted decay.
These lawsuits reflect a broader nationwide trend, with plaintiffs asserting that Suboxone’s design and method of use—dissolving a highly acidic film in the mouth daily—caused extensive dental damage. They argue that proper warnings or a safer alternative formulation could have prevented these injuries.
MDL Status and Case Management
With the motion to dismiss primarily resolved, the Suboxone MDL is now transitioning into the discovery and bellwether phase:
Discovery Orders & Case Management Developments
- New Discovery Orders (February 2025):
- The court issued new rules to streamline the collection of dental and medical records.
- A February 21, 2025, order mandates that healthcare providers accept standardized authorization forms and produce records within 30 days or face contempt under Federal Law. R. Civ. P. 45.
- The goal is to expedite the collection of evidence related to the plaintiffs’ dental history and injuries.
- Status Conferences & Trial Preparation:
- A status conference was held on February 12–13, where legal teams discussed:
- The scope of discovery (including electronically stored information custodians).
- The process for selecting bellwether cases for early trials.
- The court has directed all parties to cooperate on discovery matters, including electronic records and medical documentation.
- A status conference was held on February 12–13, where legal teams discussed:
- Upcoming Key Dates:
- An informal working session is scheduled for March 10, 2025, to address ongoing discovery issues.
- The next official status conference is set for March 11, 2025.
These procedural developments mark substantial progress in the MDL. They ensure that cases move forward efficiently while establishing a framework for early bellwether trials.
As the litigation expands, new filings are expected to continue increasing, especially as the June 2025 filing deadline approaches in certain states. The court’s focus on discovery and record collection indicates that this MDL is moving closer toward key pre-trial milestones and potential bellwether trials.
The Suboxone multidistrict litigation (MDL) in the Northern District of Ohio has expanded to 754 active cases, marking a 38% increase since December 2024. With the statute of limitations potentially expiring in June 2025, particularly in states with a three-year limit, there is an anticipated surge in filings.
Individuals alleging that Suboxone, an opioid addiction treatment, has led to severe dental issues such as tooth decay and erosion are urged to file their claims promptly. U.S. District Judge Philip Calabrese, overseeing the MDL, has indicated that the court is preparing for this influx. Due to the absence of a tolling agreement between plaintiffs and defendants, each claimant must submit their lawsuit individually before the deadline.
As of January 2025, there have been notable advancements in the ongoing Suboxone tooth decay litigation:
Case Expansion and Multidistrict Litigation (MDL):
Surge in Lawsuits: The Suboxone MDL now includes 716 active cases, following the addition of 38 new lawsuits. This represents a significant jump from just four cases filed in October, signaling a growing wave of claims against the manufacturers.
Court Proceedings and Legal Motions:
Motion to Dismiss Hearing: On December 16, 2024, Judge J. Philip Calabrese presided over arguments related to Indivior’s motion to dismiss. Indivior asserts that federal law overrides state-level claims concerning insufficient warnings. The judge’s ruling on this matter is expected to have a substantial impact on the trajectory of the litigation.
Plaintiff Documentation and Requirements:
Census Protocol Initiation: A recent case management directive requires plaintiffs who filed claims by October 7, 2024, to submit detailed information and authorizations through the Rubris Crosslink system. This process is designed to validate Suboxone Film usage and related dental damages, ensuring that claims are properly supported.
Scientific Research Highlights:
Acidity Concerns and Dental Risks: Studies have shown that Suboxone sublingual films contain highly acidic properties, which may accelerate dental erosion and decay. With acidity levels reportedly 10 times higher than tomato juice, researchers believe this could explain the widespread reports of severe dental issues among users.
These latest updates reflect the dynamic progression of Suboxone-related lawsuits as new legal arguments and scientific insights continue to shape potential outcomes and future settlements.
As of November 25, 2024, significant developments have occurred in the litigation concerning Suboxone, particularly regarding its association with severe dental issues.
Multidistrict Litigation (MDL) Status
The Suboxone lawsuits have been consolidated into a multidistrict litigation (MDL) in the Northern District of Ohio under Judge J. Philip Calabrese. As of November 2024, the MDL comprises 678 active cases, reflecting ongoing legal actions against Indivior, the manufacturer of Suboxone.
Recent Court Orders
On November 5, 2024, Judge Calabrese issued a new census order requiring all plaintiffs with cases filed by October 7, 2024, to submit key information, authorizations, and records via a Rubris Crosslink system. This order outlines steps for plaintiffs to establish and verify their use of brand-name Suboxone Film and the dental injuries linked to its use.
Allegations Against Indivior
Plaintiffs allege that Indivior failed to adequately warn users about the risk of severe dental problems, including tooth decay, erosion, and loss, associated with Suboxone. They claim that the company prioritized profits over consumer safety by not informing users of these potential side effects.
Patients are advised to consult product liability lawyers to fully understand their rights after suffering the side effects of Suboxone.
The litigation is ongoing, and further developments are expected as cases proceed through the legal system.
In a notable development in opioid litigation, pharmaceutical company Indivior agreed to pay $85 million to settle claims of healthcare fraud and antitrust violations related to its opioid-dependence drug, Suboxone. This settlement, reached just days before a five-week trial began in Roanoke County Circuit Court, is among the most prominent pharmaceutical “opt-out” settlements in recent history.
In this case, five health care benefit plans, the plaintiffs alleged that Indivior engaged in a “product hop” scheme by shifting the market from Suboxone tablets to a new film formulation in 2010. They argued that this tactic was designed to block generic competition and force healthcare plans to continue paying higher prices for the drug. A whistleblower within Indivior first exposed the scheme, which led to the plaintiffs filing suit in 2020 after opting out of the broader Suboxone multidistrict litigation (MDL).
In October 2023, a jury sided with the plaintiffs on a statute of limitations defense, ruling that their claims against Indivior were not time-barred. This verdict cleared the $85 million settlement path, highlighting ongoing scrutiny of anti-competitive practices in the pharmaceutical industry.
Earlier this month, the court held a two-day status conference for the Suboxone MDL. The focus was on putting together a plan for the upcoming bellwether trials, which will play a key role in settling the Suboxone lawsuits.
These trials show how juries might react to the main issues, like claims about flaws in the product, lack of warnings, or misleading ads for Suboxone. How these test trials turn out often steers settlement talks and gives a peek into who might be held responsible.
Legal experts agree there’s a good shot the defendants might choose to settle before these trials start to avoid unfavorable jury decisions. But setting trial dates is key to getting settlement talks going. Getting these trials scheduled could help lead to a swift, comprehensive settlement in the Suboxone litigation.
We’re closely monitoring things and will give you updates as we learn more.
A recent case management order in the Suboxone multidistrict litigation (MDL No. 3092) outlines the protocols for electronic discovery (ESI) and document production. This includes how parties will handle digital and physical documents to streamline litigation. The court emphasized the need for efficiency while safeguarding privacy and privileged information. This order aims to reduce delays and ensure compliance as the litigation grows. It reflects the importance of organization and cooperation in large-scale legal actions like the ongoing Suboxone lawsuits.
We have significant news regarding the ongoing Suboxone litigation, which could seriously help the plaintiffs’ case after the defense filed a motion to dismiss based on “implied preemption.”
In response to this motion, the Plaintiff Leadership Committee (PLC) has submitted a brief to the court that argues that Suboxone state law claims should not be dismissed under the doctrine of implied preemption unless Congress has explicitly enacted such preemption. To support this position, the plaintiffs point to two key legal developments: the recent Supreme Court decision in Loper Bright v. Raimondo and the Third Circuit of Appeals ruling in the Fosamax case.
In Loper Bright v. Raimondo, the Supreme Court’s ruling challenged implied preemption in state-level claims without further proceedings that clarify the need for the preemption; they do not automatically apply. Additionally, the Third Circuit’s decision in Fosamax supports the PLC’s argument since that court found that the lower court had made an error in applying preemption; the court ultimately sided with the plaintiffs and allowed state law claims to proceed. The main point here is that these rulings undermine the argument that statements from the FDA alone are sufficient to preempt state-based claims, the Suboxone failure to warn and design defect claims.
The PLC hopes the court will take these new rulings into consideration and allow the plaintiffs’ state law claims to remain in the case and allow them to move past the defense’s motion to dismiss.
Check back as we will continue to keep you updated on any major developments in this important part of the Subsone MDL.
After Indivior submitted a Motion to Dismiss in the ongoing Suboxone MDL, the plaintiffs promptly filed their own motion. This motion challenges Indivior’s attempt to dismiss the case, focusing on arguments related to federal preemption. Indivior’s defense centers on the claim that federal law, particularly FDA regulations, precludes the state-level liability claims brought by the plaintiffs.
The plaintiffs, however, argue that their claims are not preempted because they involve new information about Suboxone’s risks that Indivior allegedly failed to address in its product labeling. Under the FDA’s Changes Being Effected (CBE) regulations, drug manufacturers can update their labels with new warnings based on emerging risks without prior FDA approval. The plaintiffs contend that Indivior had sufficient evidence of these risks well before the FDA’s updated label in June 2022 but did not take the necessary steps to update their labeling or warn users.
Furthermore, the plaintiffs assert that their design-defect claims are valid. They argue that an alternative delivery system for buprenorphine, such as the FDA-approved injectable Sublocade, could have reduced the risks associated with Suboxone film. They believe Indivior should have considered this alternative before bringing Suboxone film to market.
This response highlights the plaintiffs’ stance that Indivior had an ongoing obligation to monitor, update, and address risks related to Suboxone, a responsibility they argue was neglected. The outcome of this legal battle will significantly impact the future of pharmaceutical litigation, particularly in cases where federal and state law intersect.
Indivior recently filed a motion to dismiss much of plaintiff Ryan Bennett’s claims. If successful, this motion could apply to all Suboxone lawsuits pending in federal court. However, the good news is that this motion to dismiss is baseless and a mere formality that occurs in every mass tort.
Indivior’s motion to dismiss is based on the doctrine of federal preemption. The doctrine of federal preemption essentially means that when federal law conflicts with state law, the court will defer to federal law or the intent of such. Essentially, Indivior contends that the label for Suboxone film was approved and thus is clearly adequate. However, a manufacturer remains responsible for their label at all times.
In this case, Indivior has access to newly acquired information that should have prompted the manufacturer to amend their warning label to inform patients and physicians that prolonged exposure could lead to serious dental injuries. Indivior clearly has access to a growing body of adverse event reports that are filed with the FDA each year. These adverse event reports consistently demonstrate tooth loss and tooth erosion and destruction of tooth enamel. However, these folks constantly suffered severe dental injuries.
Additionally, Indivior attacks Ryan Bennett’s design defect claims. The design defect claim states that there are safer alternative delivery systems than sublingual buprenorphine. Indivior claims this is mere speculation as to what the FDA would do. However, the FDA already approved Sublocade (an injectable form of buprenorphine).
We believe the Suboxone tooth decay lawsuit remains in great shape, and Judge Calabrese will almost certainly deny Indivior’s motion to dismiss. The next stage of the Suboxone lawsuit is discovery to ferret out general and specific causation.
Indivior changed the label for Suboxone film in June of 2022. Less than six months later, a research paper was published that readily demonstrates how defective the label truly is. The research letter was published in the Journal of the American Medical Association and found a substantial increase in adverse dental injuries in individuals who used sublingual buprenorphine as compared to individuals using transdermal buprenorphine or oral naltrexone.
Individuals who used sublingual buprenorphine for a prolonged period of time demonstrated a steady decline in dental health. In fact, these individuals had a greater number of tooth extractions, severe dental decay, tooth fractures, tooth erosion, and tooth loss.
This paper will serve as evidence in the Suboxone lawsuit that Indivior had access to information that should have prompted a manufacturer to change their label. In this case, Indivior should have changed the label to inform users of prescribed Suboxone film and physicians who regularly prescribe such that prolonged exposure could lead to significant dental injuries.
Dolman Russo is taking the position that the June 2022 label change does NOT serve as a basis for an existing statute of limitations. Thus, we are now reviewing potential Suboxone lawsuit claims from individuals in all fifty states.
It is our opinion that an insert is the weakest form of a warning. Indivior buried warnings or information that would educate individuals about the dangers of Suboxone film deep within an insert. Further, Indivior failed to send a “Dear Doctor” letter to physicians who regularly prescribe Suboxone films. It is our opinion that Indivior failed to adequately warn both individuals and physicians of the likelihood that prolonged exposure to Suboxone film could lead to severe dental injuries.
A joint status report was filed in the Suboxone lawsuit, which is a great indicator that both parties may have reached, or are close to, a consensus on the Suboxone MDL tolling agreement. The defense has submitted a proposed order for the plaintiffs to review and is awaiting feedback on the terms. The plaintiffs are expected to respond within a week or so. I expect that we will hear something early to mid-next week. So keep an eye out on this page, or check Lawsuit Legal News’s Subxone page for all the latest updates.
In the past month, the number of active Suboxone MDL cases dropped from 677 to 673. This decrease could be related to the Tolling Agreement both sides are working on, which would extend the filing deadlines for injured plaintiffs into 2025. The team at Dolman Law Group is anxiously waiting for the final Tolling Agreement and the MDL court’s ruling on the defendant’s motion to dismiss, described in our last update on August 1st.
Indivior, the manufacturer of Suboxone, filed a Motion to Dismiss based on several legal arguments. First, they claim federal law controls any drug design changes, so they are not responsible for changing Suboxone’s formulation after the drug was approved.
Also, the defendant argues that the FDA determined the drug was safe and effective when it was approved, and there is no new information that would require further user warnings. Indivior also raises a general argument that the plaintiffs’ allegations are not sufficient to state a legal claim against it.
We will follow this motion closely and report when the court makes a ruling.
In June, the number of Suboxone tooth decay lawsuits increased dramatically. 319 new cases were filed, raising the number of lawsuits from 358 to 677. This shows that more people are coming forward with their claims against Suboxone, and the federal case is growing quickly.
This week, the court decided against the defendants and in favor of the plaintiffs on an important causation issue. The defendants wanted to focus only on general causation issues, which would delay investigating the specifics of individual cases.
The plaintiffs argued against this, saying it would drag out the lawsuits and waste resources. But thankfully, the judge said that splitting general causation from other issues would be complicated and inefficient and that doing all the discovery together would help the Suboxone lawsuits move forward more smoothly.
The plaintiffs’ leadership committee has submitted an amended master complaint to the Court in an ongoing effort to enhance the specificity of the claims so that there is a clearer framework for the discovery phase of the MDL. The updated complaint offers a detailed plan for how plaintiffs will substantiate their allegations against Indivior. As the discovery phase gets closer, this move is all about streamlining the process and strengthening the plaintiffs’ position.
The litigation is waiting on Judge Calabrese’s decision on the general and specific causation discovery debate, which has been a major part of the case so far.
On June 10th, Judge Calabrese heard arguments from both sides about whether these lawsuits should first focus on general causation evidence before addressing specific causation issues.
The defendant Indivior wants to limit the discovery of relevant evidence to the general causation question: Does Suboxone use cause dental problems? As a result, discovery related to specific causation evidence—how each plaintiff’s use of Suboxone led to their dental issues—would be delayed.
The mass tort team at Dolman Law Group believes Indivior simply wants to drag out these cases, frustrate the plaintiffs and their lawyers, and run up litigation expenses for the law firms. and pressure injured Suboxone users into giving up. We hold firm that when the FDA changed the warning label on sublingual Suboxone in 2022, there was a solid causal link between using sublingual Suboxone and adverse dental conditions. Hopefully, the judge will deny the defendant’s request and allow the MDL to proceed on both causation issues.
Also, plaintiffs’ cases are now divided into two lists – those filed by plaintiffs in New Jersey, Delaware, and Virginia and those filed by plaintiffs from all other states. For federal jurisdiction, the plaintiff and defendants must have jurisdictional diversity. This means the parties cannot “reside” in the same state. Since the named defendants in these cases are located in New Jersey, Delaware, and Virginia, plaintiffs from those states would normally have to file their lawsuits in state court, and they wouldn’t qualify for the federal MDL.
Under the recently entered tolling agreement, plaintiffs from those three states now have more time to decide whether to file a state or federal lawsuit and will not lose their legal rights due to the passage of time.
Plaintiffs in the Suboxone MDL are urging the court to reject a defense proposal to split the litigation into two phases.
This would hold off on initial discovery into individual claims and instead focus on general causation. Plaintiffs argue this would unnecessarily delay the claims and justice for the victims.
As you likely know by now, the JPML centralized the lawsuits to coordinate pretrial proceedings and early bellwether trials.
However, Indivior’s proposal to bifurcate discovery would defeat some of the purposes of an MDL by slowing down the case, delaying case-specific discovery, and wasting time on proving general causation.
Plaintiffs argue that this approach assumes that the defendants will win, which we believe is unlikely given the extensive scientific evidence linking Suboxone to tooth decay.
On May 24, plaintiffs filed an opposition brief stating that phased discovery would unnecessarily prolong the MDL and waste resources.
They also noted that most MDL courts avoid bifurcation because of this very reason. Judge Calabrese has ordered the defense to respond by May 31 and has scheduled oral arguments on the bifurcation/phased discovery proposal for June 6.
Recently, the Judge overseeing the Suboxone MDL issued two important Case Management Orders related to protected and privileged information. Here’s a brief overview of each order.
Case Management Order No. 5: Designation and Handling of Protected Information
This order outlines how to handle protected information in the Suboxone lawsuit. It defines “Confidential Information,” “Highly Confidential Information,” and “Protected Data.” It specifies who can access these materials and how to label and manage them. It includes procedures for marking these documents, handling any errors, protecting personal data, and addressing breaches or the need to share confidential information in open court.
Case Management Order No. 6: Evidence Rule 502(d) and Privileged Materials
This order focuses on handling privileged materials in the Suboxone MDL. It applies Rule 502(d) of the Federal Rules of Evidence, which protects a person from waiving confidentiality when they share privileged information during the case. This rule allows parties to share information without losing legal protections like attorney-client privilege. This ensures any purposely or mistakenly disclosed information remains protected.
Both Case Management Orders No. 5 and No. 6 will remain in effect until the court modifies or terminates these orders.
On May 13, Suboxone’s defense team officially rejected the tolling agreement, which would have allowed plaintiffs to file lawsuits after the statute of limitations expired so they could vet the cases before adding them to the MDL. This would not have allowed new claims to come in after the deadline but would have allowed cases that occurred before the deadline to be delayed in filing. It was not a huge ask, but Indivior wants to pay as few people as possible, so it’s in their best interest to have cases refused due to a technicality.
As we mentioned, the tolling agreement would have also helped the Court and maintained the integrity of the MDl. Perhaps because of this, Judge Calabrese adopted a procedure we last saw used in the Xarelto case, where the plaintiffs must submit a master list by the deadline and then file all their cases at a later deadline.
For states with a two-year statute of limitations, the cut-off date for Suboxone lawsuits is June 14, 2024. Therefore, the Judge ruled that the plaintiffs’ lawyers must file a master MDL list of all individual plaintiffs by that date. The defendants will then have until July 1, 2024, to file a motion for severance.
This approach aims to reduce the court’s burden from a sudden influx of cases and provide a structured process for addressing the statute of limitations issue. We will provide you with more details after the next hearing.
Suboxone’s lawyers have finally explained their refusal to consent to a tolling agreement in the MDL. Today, the defense filed an opposition brief that made clear that a tolling agreement is not in their best interest. Basically, they recognized the benefits of the tolling agreement, but since it’s not in their interest, they would prefer the SOLs to expire in 2-year states.
In their brief, they stated, “Defendants would get no benefit other than purported proof of use of Suboxone film, untethered to causation and purported damages.”
Plaintiffs argued that a tolling agreement would be fair to consumers and prevent Indivior from having to litigate in multiple jurisdictions due to varying statutes of limitations. Despite our expectations that Indivior might agree to avoid multi-jurisdictional litigation, it is now clear they will not.
This decision will push more cases toward state courts rather than the consolidated federal MDL, more or less defeating its purpose. The Judge may have something to say about this since it will so greatly affect the Court’s time and money too.
We gained a little more insight into the case’s progress at the last status conference for the Suboxone lawsuit MDL. Jude Calabrese held the latest status conference in the Suboxone lawsuit last week in the United States District Court for the Northern District of Ohio. Arguments were preferred in chambers by both parties concerning the order in which discovery wiki be conducted.
Indivior is aggressively advocating for discovery solely on general causation before both parties turn their attention to specific causation. This is nothing more than a glorified stall tactic by the defendant manufacturer. This would result in adding a year to perhaps eighteen months to the duration of the Suboxone tooth decay MDL.
Plaintiffs have the burden of illustrating through sound scientific evidence that prolonged exposure to sublingual buprenorphine medications causes tooth loss, among other serious dental problems that require extensive dental procedures.
As previously referenced in our last update, the FDA unilaterally forced Indivior to change its warning label in June of 2022. The change in warning label was prompted by an alarming number of adverse event reports filed by sublingual Suboxone users with the FDA, many of whom complained of tooth loss. There are also several peer-reviewed and published medical studies that illustrate a causal link between prolonged use of sublingual buprenorphine and serious dental problems.
We believe general causation will not be a difficult burden for plaintiffs to meet in the Suboxone lawsuit. Specific causation, on the other hand, will be much more difficult. Plaintiffs will have the burden of showing that a specific individual’s use of Suboxone sublingual strips leads directly to their tooth loss. It is our opinion that Judge Calabrese will opt to move this lawsuit in a prompt manner and focus the litigation and discovery on whether plaintiffs can prove specific causation
Suboxone lawyers and defense attorneys from Indivior also discussed the potential for a tolling agreement at the last hearing. A tolling agreement would effectively suspend all existing statutes of limitations until a specified period of time or event occurs. This would allow plaintiffs to perfect their lawsuits and investigate any potential issues prior to filing a Suboxone lawsuit. Many lawsuits in states with a two-year statute of limitations were rushed into litigation to preserve the rights of their clients.
A tolling agreement would preclude problematic lawsuits from being filed and prevent a waste of judicial economy. In turn, Indivior’s defense counsel would no longer be forced to answer every last Suboxone lawsuit filed. This issue is going to be briefed by lawyers from both sides over the coming weeks.
Indivior, the company that manufactures Suboxone, wants the Judge to limit the current litigation to only focus on whether the drug can be scientifically linked to dental problems instead of considering whether specific plaintiffs can establish a link between their individual Suboxone use and tooth decay.
At Dolman Legal Group, we believe this tactic is simply meant to delay the litigation and frustrate the plaintiffs who need financial compensation to pay for their dental care. The theory is that, over time, some injured plaintiffs may eventually become desperate for money and accept any settlement offered to finalize their claims.
Indivior cites other Multidistrict Litigation situations in which plaintiffs were first required to provide proof that the product could harm users. However, the Suboxone MDL is different because Indivior allowed the FDA to place a warning on Suboxone related to tooth decay in 2022 without a fight. The fact that Suboxone now carries a warning about dental issues shows that Indivior and the FDA acknowledge a scientific link between using Suboxone and severe tooth decay.
A warning is usually only added to a drug label after enough users report medical issues and/or scientific studies corroborate the alleged problems. Rather than wasting time discussing general causation, our legal team hopes the court will consider both general and specific causation at the same time to resolve these matters as quickly as possible.
As we previously predicted, the Suboxone lawsuit has grown quickly because of the statute of limitations deadlines that affect certain states. Many of the law firms on the Suboxone plaintiff’s steering committee have been furiously filing lawsuits to preserve the rights of individuals residing in states with a two-year statute of limitations. Unfortunately, injured Suboxone users who reside in states with a two-year statute of limitations will lose their right to file a lawsuit in June 2024. Many law firms are no longer accepting those cases.
We anticipate the Suboxone lawsuit will inevitably exceed 500 plaintiffs by mid-summer, placing pressure on Indivior to reduce their overall exposure and potentially settle these cases over the next two years.
Currently, we have limited our intake to clients who have lost three or more teeth or had three or more tooth extractions. While most prospective clients contacting our firm have begun to suffer severe tooth decay, we believe tooth loss is required for a sustainable claim.
The Suboxone Multidistrict Litigation (MDL) was formed in February of this year and already contains 86 lawsuits filed by people who suffered severe tooth damage after using Suboxone sublingual strips. As we mentioned below, in June 2022, the FDA required Indivior, the maker of Suboxone, to change the warning label on this product. As a result, people who were injured by Suboxone and live in a state with a two-year statute of limitations must file their Suboxone lawsuit before June 2024. Unfortunately, we are too close to this deadline to accept any more cases from people living in two-year deadline states.
At Dolman Law Group, we have also narrowed the scope of the cases we can accept to make sure the strongest cases join the pending MDL. We are currently only working with Suboxone users who have suffered the loss or extraction of three or more teeth as a result of these strips. Tooth loss has become the driving factor behind Suboxone lawsuits. The overarching goal is not to flood the Northern District of Ohio with Suboxone product liability lawsuits alleging injuries that may have a number of confounding factors or are not considered significant by the defendant manufacturer.
Michigan Governor Gretchen Whitmer just signed a bipartisan bill into legislation that repeals a law that previously provided drug makers with absolute immunity from liability. Michigan had the only law in our nation that shielded drug manufacturers from product liability claims.
Michigan has a three-year statute of limitations for product liability claims. Thus, we will be accepting Michigan Suboxone tooth decay claims until late May 2025. We are now cutting off signing up Suboxone tooth decay cases from individuals residing in States with a two-year statute of limitations for product liability claims. Since the FDA forced Indivior to change its warning label for prescription Suboxone film in June of 2022, we are simply too close to the deadline expiring in two-year states to feel comfortable taking such cases.
Judge Calabrese held the first status conference of the Federal Suboxone lawsuit wherein he appointed a Plaintiff’s Steering Committee with eighteen members, including Stanley Gipe from Dolman Law Group. We anticipate coordinated discovery will soon commence.
Please note that Judge Calabrese has scheduled the next status conference hearing for April 16, 2024, at 2pm. Further, Judge Calabrese requested that both parties submit an agenda for the next status conference by April 11, 2024.
A leadership slate for the plaintiff’s steering committee on the Suboxone tooth decay lawsuit has been submitted to Judge Calabrese. We are proud to announce that R. Stanley Gipe of Dolman Law Group/Dolman Russo is among the lawyers who will almost certainly comprise the plaintiff’s steering committee.
The initial status conference in the Suboxone lawsuit is scheduled for Thursday, March 7, 2024, at 9 a.m., in front of the honorable Judge Philip Calabrese in the United States District Court for the Northern District of Ohio.
We anticipate Judge Calabrese will likely approve the plaintiff’s proposed leadership slate and begin coordinating discovery to commence between plaintiffs and defendants in the Suboxone lawsuit.
It is worth noting that we are quickly approaching the expiration of a statute of limitations for plaintiffs residing in states with a two-year statute of limitations for product liability claims. The warning label on Suboxone film was changed back in June of 2022.
Two new Suboxone lawsuits have joined the Suboxone MDL pending before Judge Philip Calabrese in the Northern District of Ohio. First, a Kentucky man who has used Suboxone sublingual films since 2011 alleges he was never warned about the potential harm he would suffer until 2023. He is requesting compensation for extensive dental expenses, pain and suffering, and irreversible damage due to Suboxone use.
The plaintiff is requesting compensation for extensive dental expenses, pain and suffering, and irreversible damage due to Suboxone use.
The second Suboxone tooth decay lawsuit was filed by an Ohio man who also experienced extensive dental issues requiring many painful dental treatment procedures. He claims his doctor was not aware of potential risks associated with sublingual buprenorphine, so he received no warning about Suboxone use.
We are presently representing hundreds of individuals who were prescribed Suboxone films and subsequently developed severe dental injuries, including severe tooth decay, tooth loss, tooth fractures, dental caries, and gum infection, among other oral health issues.
It’s official… federal Suboxone lawsuits are consolidated into an MDL (multidistrict litigation). No oral arguments were heard at the JPML hearing since it wasn’t necessary. Both sides agreed to consolidate the cases into an MDL before the hearing. Federal Judge J. Philip Calabrese from the Northern District of Ohio was appointed to oversee Suboxone litigation and all federal claims against Indivior for tooth decay and other serious oral health issues.
Apparently, Indivior, the company that manufactures Suboxone, knew about the potential risk that users could experience tooth decay since it is mentioned in the prescribing information provided to doctors. However, it appears they didn’t think it was important enough to include in the medication guide, which warns patients about the potential side effects of a specific drug. We expect to learn more about the MDL decision after January 25.
A new Suboxone tooth decay plaintiff, Lindsay Haddad, has emerged as one of the thousands of Americans who suffered profound dental injuries after using the medication to reduce her dependence on opioids.
A new Suboxone tooth decay plaintiff, Lindsay Haddad, has emerged as one of the thousands of Americans who suffered profound dental injuries after using the medication to reduce her dependence on opioids. Her product liability lawsuit claims that Indivior effectively deprived the medical community of the information they needed to care for their patients properly, relied on insufficient testing to vet their medication, did not accurately inform consumers about the risks of using Suboxone, and manufactured the medication based on a defective design.
Her Suboxone lawsuit claims Indivior effectively deprived the medical community of the information they needed to care for their patients properly, relied on insufficient testing to vet their medication, did not accurately inform consumers about the risks of using Suboxone film, and manufactured the medication based on a defective design.
A Cuyahoga County woman has become the most recent plaintiff to file a Suboxone tooth decay lawsuit alleging the drug manufacturer Indivior concealed the risks of the medication from patients and providers.
A Cuyahoga County woman has become the most recent plaintiff to file a Suboxone tooth decay lawsuit alleging the drug manufacturer Indivior concealed the risks of the medication from patients and providers. Her complaint describes the significant tooth decay she experienced as a result of using Suboxone, which includes an active ingredient known to create the conditions for enamel erosion. Including this claim, over 15 plaintiffs have taken legal action against Indivior in federal court, although there could be hundreds more eligible plaintiffs across the country. Given the similarity of the Suboxone tooth decay claims and the potential for expansion, the parties have asked to consolidate the cases into multidistrict litigation (MDL). The defendants have suggested centralizing the Suboxone tooth decay lawsuits in the Northern District of Ohio, where Cuyahoga County is located.
Given the similarity of the Suboxone tooth decay claims and the potential for expansion, the parties have asked to consolidate the cases into multidistrict litigation (MDL). The defendants have suggested centralizing the Suboxone tooth decay lawsuits in the Northern District of Ohio, where Cuyahoga County is located.
More lawsuits are expected as the number of Suboxone tooth decay lawsuits continues to rise, with hundreds of potential cases being investigated by lawyers nationwide. This trend suggests that the issue of Suboxone-related dental problems is not likely to go away any time soon. As of January 3, 2024, there have been no announced settlements in any Suboxone tooth decay lawsuits.
Keep in mind that this class action lawsuit is in its infancy. A Suboxone settlement is unlikely to occur in 2024. We anticipate that all federal Suboxone tooth decay lawsuits will be consolidated by the Judicial Panel on Multidistrict Litigation (JPML) over the coming months.
A settlement in the Suboxone lawsuit will become more likely as discovery proceeds and plaintiff lawyers are able to demonstrate their experts can causally link the use of sublingual buprenorphine.
More studies are emerging that support a link between Suboxone use and an increased risk of tooth decay. These studies seem to show that the drug changes the oral bacteria balance and decreases saliva production in the user’s mouth, both of which can contribute to dental problems. Although the link between Suboxone and tooth decay is becoming clearer, the specific ways in which Suboxone affects the teeth and gums are still not exactly known. Further research is being done to understand the long-term effects and how dosage variations and oral hygiene techniques might curb these negative side effects.
The Judicial Panel on Multidistrict Litigation (JPML) has scheduled their next hearing for January 25, 2024, in Santa Barbara, California, where they will hear arguments from both plaintiff and defense lawyers on whether all pending Suboxone teeth lawsuits pending in Federal Courts throughout the United States, should be consolidated in one jurisdiction as part of multidistrict litigation (MDL). We believe the JPML will consolidate the Suboxone class action lawsuit in the Northern District of Ohio.
The MDL motion alleges all of the claims filed against the drug manufacturer Indivior related to Suboxone injuries have similar facts and legal arguments, including the reason Suboxone is prescribed—for people struggling with opioid use disorder (OUD). However, Suboxone’s film is acidic and connected to tooth erosion and dental decay, and Indivior should be held liable for manufacturing and selling Suboxone when it knew or should have known about these potentially dangerous side effects and harming users.
Plaintiffs also assert that since 2007, patients have been reporting dental issues, and research starting in 2012 has shown a potential connection between taking Suboxone sublingually (under the tongue) as prescribed and severe tooth decay. Also, even though it knew about these potential risks, Indivior failed to change the drug or warn about the risks. This is at the heart of allegations in Suboxone lawsuits.
Plaintiffs’ lawyers petitioned the U.S. Judicial Panel on Multidistrict Litigation (JPML) to consolidate all Suboxone lawsuits that have been filed in Federal Courts throughout the country into one jurisdiction.
The plaintiffs have asked the JPML to consolidate Suboxone lawsuits into the Northern District of Ohio. Considering that plaintiff David Sorensen filed the first Federal lawsuit linking the use of sublingual Suboxone to tooth decay in the Northern District of Ohio, we feel this is a likely jurisdiction for the Suboxone teeth lawsuits.
Suboxone lawyers are satisfied with the science (an easy causal link between Suboxone film use and issues related to dental health) and the back story on Indivior being a bad actor.
Four new Suboxone tooth decay lawsuits were filed against Indivior in federal courts over the past month. Each lawsuit alleges a physician prescribed Suboxone film to treat opioid addiction. Neither the plaintiff or his/her physicians were adequately informed of the risk associated with sublingual Suboxone film per allegations contained within each lawsuit.
Indivior now faces multiple avenues of litigation that they must now fight off. On one hand, Indivior must defend a False Claims Act lawsuit accusing the company of an illegal kickback scheme. At the same time, there is an ever-growing number of lawsuits alleging the use of Suboxone (film form) led to advanced tooth decay.
It is important to know that Indivior has denied any wrongdoing and is fighting these lawsuits in court. However, the sheer number of people with tooth decay who took Suboxone, along with scientific evidence, means there’s a significant risk that Indivior will be found liable for their negligence.
Can I Sue Suboxone for Tooth Decay?
Yes. If you used Suboxone film before the manufacturers updated their warning label in June of 2022, and you suffered severe tooth decay, then we would be honored to pursue a claim on your behalf. The general allegation is the manufacturer of Suboxone failed to warn users of a known danger or defect associated with using their product.
We believe Indivior is a bad actor and rushed sublingual buprenorphine (sublingual version of Suboxone) to market in order to avoid generic competition to Suboxone tablets as the patent was about to expire.
Further, it is our belief that Indivior was well aware of the acidity levels of sublingual Suboxone strips and the impact it could have on tooth enamel, yet failed to inform the medical community and consumers of the potential for serious dental injuries. These allegations are common in the Suboxone class action lawsuit. The vast majority of our clients have sustained injuries that require extensive dental treatments
Keep in mind that Reckitt Benckiser (parent company to Indivior) previously entered into a settlement with the U.S. government, where they paid $1.4 billion to resolve both civil and criminal charges concerning how Suboxone was marketed. The problem is that the drug manufacturer and its parent company were caught red-handed suppressing generic competition for Suboxone (their opioid addiction medication).
New Suboxone Lawsuits
On February 2, 2024, all individual lawsuits filed in Federal Court were consolidated before Judge Philip Calabrese in the United States District Court for the Northern District of Ohio. The purpose of consolidating Suboxone tooth decay lawsuits is to coordinate pretrial proceedings.
However, our law firm is only taking new Suboxone lawsuits in States with at least a three-year statute of limitations. The FDA unilaterally changed the warning label on prescription Suboxone film in June 2022. We will soon file all of our Suboxone lawsuits for clients residing in states with a two-year statute of limitations. After June of 2024, any new Suboxone lawsuit must be filed only on behalf of individuals residing in states with three, four, or five-year statute of limitations.
Can I Still File a Suboxone Lawsuit In My State?
We have been getting a lot of calls from people asking about the statute of limitations in their state and whether they can still file a Suboxone Lawsuit.
The information regarding the statute of limitations and which states are eligible can be confusing and contradictory, especially because of the different legal theories, like “failure to warn”.
Since Indivior added a warning label in January 2022 to Suboxone alerting patients to oral health risks, the statute of limitations for filing lawsuits in states with a two-year limit has expired as of January 2024.
However, we are still accepting Suboxone tooth decay claims in states with filing limits of three, four, and five years.
Here is a list of which states we are accepting cases from:
- Florida
- Arkansas
- Maine
- Maryland
- Michigan
- Minnesota
- Massachusetts
- Missouri
- Montana
- New Hampshire
- New York
- New Mexico
- North Carolina
- North Dakota
- Rhode Island
- South Carolina
- South Dakota
- Vermont
- Washington
- Wyoming
- Wisconsin
If you live in one of the above states and experienced serious dental issues (tooth loss, fractures, extractions, or severe tooth decay) while using Suboxone sublingual films, call us immediately. You may be entitled to compensation for the extensive dental treatments required to restore your oral health.
What is Suboxone?

Suboxone is a prescription medicine used to help people who are struggling with opioid addiction and dependency. The medication is administered as a thin strip of film that is taken sublingually (placed under the tongue), where it dissolves and is then absorbed by the body. This prescription drug is used solely for opioid addiction.
Patients prescribed Suboxone typically are struggling with opioid use disorder (OUD). Suboxone does provide real relief for people who are trying to fight this medical condition. Still, it is a medication intended to be used as part of a greater program of detoxification and treatment utilizing other forms of help.
Some people may experience side effects when taking Suboxone, like nausea, headache, and constipation. However, it’s crucial to talk to a doctor about anything that is concerning you, especially if it involves tooth decay.
How Suboxone Works
By utilizing the two main ingredients, buprenorphine and naloxone, Suboxone can deliver its useful effects that help treat opioid use disorder. Suboxone contains one part naloxone to four parts buprenorphine.
Buprenorphine in Suboxone
Buprenorphine is a partial opioid agonist that provides a limited version of opioid effects and can curb withdrawal symptoms and cravings without delivering the same potency as opioids like heroin and certain pain prescriptions.
Opioid agonists such as heroin, morphine, and pain prescriptions activate nerve cells in the brain by attaching to proteins known as opioid receptors that block pain and release endorphins that cause sensations of pleasure and euphoria in what is known as the “opioid effect.”
Buprenorphine is only a partial opioid agonist, which means that it does attach to opioid receptors like other opioids, but it only partially activates the receptors. As a result, it does not create the same potency as the “opioid high”. So buprenorphine gives those with OUD an alternative to their desire for opioids that tricks the brain into thinking it is getting the opioids it desires to curb withdrawal symptoms.
Buprenorphine also has the added benefit of adhering to opioid receptors, so if a user relapses, the buprenorphine will interfere with the other opioids with what is called a “ceiling effect.” The full opioid agonists in the other drugs taken in relapse will not have the same potency since the buprenorphine bonded to the opioid receptors will interfere with their activation.
This also helps to reduce the likelihood of overdose deaths, although some who relapse may attempt to use larger doses to overcome the ceiling effect, which is extremely dangerous.
Naloxone in Suboxone
Naloxone, also known as Narcan, is an opioid antagonist that blocks the activation of opioid receptors and blocks any euphoric effects. When Suboxone is taken sublingually as intended, its naloxone does not activate. However, if patients attempt to abuse Suboxone by injecting it intravenously since buprenorphine has more potent effects taking it that way, the naloxone activates and blocks opioid receptors, leading to a lack of opioid effect while the patient still suffers withdrawal symptoms.
Suboxone and Tooth Decay
Tooth decay has become a concern for people who are prescribed Suboxone sublingual film. Many users have reported (following long-term use) experiencing severe dental injuries and dental issues, including:
- Severe Tooth Decay: One of the most significant issues reported is severe tooth decay, which can result in cavities and damage to the tooth structure. This damage may require:
- Tooth extraction
- Root canal treatment
- Crown or crown replacement
- Tooth Erosion: Suboxone use has been linked to erosion of tooth enamel, making teeth more susceptible to decay and sensitivity. The following are some injuries caused by Suboxone-related tooth erosion.
- Dry Mouth: Some individuals on Suboxone experience dry mouth as a side effect, reducing saliva production. Saliva helps protect teeth from decay, so reduced saliva flow can contribute to dental problems, including tooth loss.
- Gum Problems: Suboxone may also lead to gum issues, including gum inflammation and periodontal disease.
- Infections: The damage done to the teeth and gums by Suboxone can potentially lead to infections. These infections are typically treated with medication and/or certain procedures, but complications are possible.
What Evidence Is There That Suboxone Causes Tooth Decay?
The potential link between the sublingual film version of Suboxone use and tooth decay is based on anecdotal evidence and studies that have observed concerning relationships between Suboxone and rates of tooth decay. However, it’s important to know that more research is needed to establish a direct relationship between taking Suboxone and tooth problems. We will require dental records from before your use of Suboxone to illustrate the damage caused by the sublingual film.
Patient Reports of Suboxone-Related Dental and Oral Injuries
Many individuals have reported experiencing dental problems, including tooth decay while using Suboxone. Suboxone films have an acidic makeup that was not adequately tested for potentially causing dental erosion. These reports have been a significant factor in raising awareness about the issue. If you have suffered from dental and oral health issues after using Suboxone, then do not hesitate to report your injuries. You will be asked to provide your lawyer with records of your Suboxone usage, dental care, and evidence of dental injuries and/or gum disease.
Suboxone’s Acidic Nature Causes Dental Damage
Suboxone strips have an acidic pH, which can potentially contribute to enamel erosion and decay over time if good dental hygiene is not followed. Suboxone has a pH of 3.4 on the pH scale, which is used to determine the basicity or acidity of substances ranging from 0 to 14, with 0 being highly acidic and 14 being highly basic.
Either extreme on this scale is dangerous, but to give some perspective, 0 on the pH scale is acidic on the level of battery acid, and 14 is drain cleaner. Both extremes are highly corrosive and dangerous, with 7 at the middle of the scale being the most neutral, like water.
Suboxone strips have a pH of 3.4, which is somewhere between vinegar and orange juice. On average, your mouth maintains a slightly acidic pH level between 6.2 and 7. Regular use of acidic Suboxone strips can lower the pH of the mouth and cause the steady erosion of the enamel of the teeth
Dry Mouth Side Effect of Suboxone
Dry mouth, also known as xerostomia, is a known side effect of Suboxone. Since the medicine is taken sublingually (under the tongue) it reduces saliva production which can contribute to tooth decay since saliva plays a vital role in protecting our teeth by neutralizing acids and preventing the buildup of harmful bacteria.
When those taking Suboxone experience dry mouth symptoms, then that can exacerbate the already acidic effects of the medicine and further lower mouth pH, leading to tooth decay.
Medical Studies of Suboxone Conclude It Increases Risk of Dental Injuries
Some preliminary studies have suggested a possible association between opioid medications like Suboxone and dental problems, but further research is needed to establish a clear causal link.

One study published in the Journal of Clinical Pharmacy and Therapeutics in 2016 found that people taking Suboxone were more likely to experience tooth decay than people not taking the medication[1]. The study also found that Suboxone was associated with an increased risk of xerostomia, or dry mouth, which is a known risk factor for tooth decay.
A second study [2] entitled “Association Between Sublingual Buprenorphine-Naloxone Exposure and Dental Disease” found a significantly increased risk of adverse dental health outcomes associated with sublingual buprenorphine use.
Another study, published in the peer-reviewed Prim Care Companion CNS Disord, found that people taking Suboxone had a higher prevalence of dental caries or cavities than people not taking the medication. The study also found that Suboxone was associated with an increased risk of tooth erosion, which is a condition that can weaken tooth enamel and make it more susceptible to decay.
A more recent study, published in the Journal of Addiction Medicine in 2022, found that people taking Suboxone had a higher risk of dental problems than people taking other medications for opioid use disorder (OUD). The study also found that the risk of dental problems was highest among people who had a history of dental problems before starting Suboxone.
While these studies suggest that Suboxone may increase the risk of dental problems, it is important to note that more research is needed to confirm these findings. However, the FDA has taken notice. In January 2022, the FDA issued a warning [3] that buprenorphine medications that dissolve in one’s mouth significantly increase the risk of dental injuries. It is vital for physicians to contemplate these potential risks when prescribing suboxone.
Additionally, it is important to weigh the potential risks of dental problems against the risks of being addicted to opiates, like heroin or street pills, and what that can do to your life.
Learn more: Florida Prescription Opioid Crisis Statistics
FDAs Response to Suboxone Tooth Decay
In 2022, the FDA warned about the risk of dental problems linked to buprenorphine (Suboxone). The FDA report on Suboxone stated that some individuals experience significant oral problems such as tooth decay, cavities, infections, and tooth loss.
Reports show these issues have occurred in people who have never had dental problems in the past and who have great dental hygiene. The FDA has determined that based on a body of medical research and a database of adverse events, there is a link between the sublingual form of Suboxone that dissolves in the mouth and the development of severe tooth decay and other significant dental problems.
As a result, the FDA has required manufacturers to add a warning about these dental risks in the prescribing information and the patient Medication Guide.
These medical issues can be painful and very expensive to fix. That is why you should be to an experienced defective drug lawyer like Dolman Law Group, who has taken on some of the biggest drug manufacturers in the world and won.
The link between Suboxone and tooth decay has raised questions about the medication’s side effects. Some users have noticed a connection between Suboxone use and dental problems for years but weren’t sure if they were linked or what they could do about it. The medical journal JAMA published a research report in December 2022 that sublingual film forms of Suboxone are two times more likely to cause serious dental health issues than regular tablets.
While the exact cause is still under investigation, these reports have led product liability lawyers like Dolman Law Group to look at holding these manufacturers accountable for potential health risks that were not properly conveyed to consumers in warning labeling. We believe it is wise to consult with medical professionals before making any determinations regarding your health.
Allegations Against Indivior in Suboxone Tooth Decay Lawsuit
The victims who are suing Suboxone and their legal team (known as plaintiffs) claim that Indivior, the maker of Suboxone, knew about the medication’s potential for causing tooth decay and other dental problems due to the acidic formulation of Suboxone sublingual tablets and films (the medication is in a thin strip or tablet and is placed on the cheek [buccal administration] or under the tongue [sublingual administration] for 30 minutes) and did not take adequate steps to warn users about this risk or to prevent it from becoming a problem.
Indivior’s failure to warn and protect patients is the main point of the Suboxone lawsuits.
As early as 2007, adverse event reports were being reported to the FDA, and studies in medical journals were pointing to a possible link between Suboxone tablets and films and severe dental decay.
Between 2007 and 2021, at least 136 adverse events related to oral health issues associated with the use of Suboxone were reported to the FDA and the manufacturer. Indivior was aware that people being prescribed their medication were experiencing very specific and recurring issues but chose not to act until much later.
Around 2010, Suboxone tablets were reaching the end of their patent protection. But manufacturers of Suboxone—at the time Reckitt Benckiser, but later a subsidiary called Indivior broke away and took Suboxone with them—developed the film version of the medication to mitigate competition with generic versions of the tablet.
To do this, the defendants gradually raised the price of Suboxone tablets and kept the price of films steady in order to switch patients over to the new films. This later led to antitrust violations and criminal convictions of senior executives.
This is to say that Reckitt and later Indivior specifically pushed films, and after they got a final warning by the FDA in January 2022 about serious dental issues being reported because of Suboxone’s sublingual delivery method, they added a warning. But at this point, patients had been suffering for years with tooth decay, gum disease, and other issues.
So in short, people are suing because they knew or should have known about Suboxone’s potential for tooth decay and failed to warn doctors and the public about it.
Indivior’s Past Legal Trouble Could Be Linked to the Suboxone Tooth Decay Lawsuit
Indivior decided to pay $385 million in October to settle claims that it unfairly maintained a monopoly over Suboxone. Earlier in 2023, they had already agreed to pay $102.5 million to multiple US states for affecting state healthcare costs and $30 million to settle with health insurers over similar issues.
The allegations in these previous lawsuits suggest Indivior rushed Suboxone’s film version to market just as their patent on Suboxone tablets was about to run out. No patent meant other drug makers could produce their own version of Suboxone, and poof, profits go away.
But in an illegal move known as product hopping, Indivior has been accused of coming up with a new type of Suboxne that was supposedly “better” and “more effective”, thus steering the market back into their pockets.
This medication was Suboxone sublingual films. Which they happened to get on the market just in time as their patent on the tablet form of Suboxone ran out. Unfortunately for thousands of Americans, Indivior either failed to notice or failed to warn patients that the sublingual films can cause severe tooth decay, which is why they are facing the Judge again now.
Indivior settled these monopoly lawsuits without admitting guilt, ending 10 years of legal battles, possibly to free up their lawyers for this fight.
Suboxone Dental Decay Lawsuit – Symptoms
Tooth decay is only one of many possible negative symptoms that can occur when using Suboxone, with patients also possibly suffering symptoms (potential side effects) such as:
- Nausea and Vomiting
- Headache
- Sweating
- Numb Mouth
- Permanent tooth decay/dental decay
- Broken teeth
- Gum Injuries
- Oral Infections
- Tooth Damage or Damaged Teeth
- Painful Tongue
- Dizziness and Fainting
- Problems with Concentration
- Degradation of Oral Health
- Chronic Pain
- Fractured Teeth
- Gum Recession
- Dry Mouth – Decreased Salivary Flow
The issue is that there was adequate warning of these negative symptoms for patients and doctors considering Suboxone for treatment but no warning of tooth decay that Suboxone can also cause. Indivior did not include warnings about potential tooth decay caused by Suboxone until 2022.
Suboxone was approved by the Food and Drug Administration (FDA) in 2002 for OUD treatment, meaning that patients were deprived of essential information that they had the right to know about regarding a harmful Suboxone side effect for about 20 years.
The product liability lawsuits filed against Indivior are focused on the fact that Suboxone’s labeling did not include warnings of tooth decay. Lawyers are suing the manufacturer for negligence in not providing that info and not simply because suboxone has a negative side effect of tooth decay alone.
Maintaining Oral Health and Recognizing Early Signs of Tooth Decay
Taking care of your teeth is important; we all know this. It’s the only set you get. But it’s even more important when you are on Suboxone, which is why we have covered some important information below. Additionally, knowing what to look for when it comes to tooth decay while you are on Suboxone is extremely important.
Taking Care of Your Teeth While Taking Suboxone
Keeping your teeth healthy while taking Suboxone is important. Keep in mind how Suboxone causes tooth decay, like dry mouth, acid, and bacteria growth, and make sure to prevent as many of these side effects as possible.
- Brush thoroughly twice a day: Use a soft-bristled toothbrush and toothpaste to protect your teeth from decay. Soft bristles will help prevent further damage, and the fluoride in the tooth will help combat the effects of acid on your tooth enamel.
- Floss daily: Flossing removes plaque and food particles from places your toothbrush can’t reach. This is crucial since Suboxone can reduce saliva flow, making your mouth more prone to bacteria buildup and more likely for food to stick between your teeth.
- Increase water intake: Dry mouth is a common side effect of Suboxone, so staying hydrated will help keep your mouth moist and protect against tooth decay. Keep a bottle of water with you so that you can always keep your mouth wet.
- Eat a balanced diet and limit acidic drinks: Foods high in sugar and acidic beverages can further damage your tooth enamel, especially since Suboxone contains sweeteners and is acidic. So if you’re a soda or coffee drinker and on Suboxone (which, face it, you probably are), make sure you use that water from step three to rinse your mouth.
- Visit the dentist regularly: Whether you are taking Suboxone or not, you should obviously visit your doctor at least twice a year. But if you are taking Suboxone, you should make sure to tell your dentist so they can keep an extra close watch on your oral health. They may also be able to offer specific advice or treatments to counteract Suboxone’s effects on your teeth, like a specialized mouthwash to help with dry mouth.
Rinse Your Mouth After Taking Your Subxone Films
The directions for sublingual Suboxone films instruct the user to put the medicine in their cheek or under the tongue and to wait for it to dissolve; based on studies and anecdotal reports, this usually takes around 10-15 minutes. The patient is instructed to not drink or eat anything for 30-45 minutes after the medicine dissolves.
Suboxone’s instructions also mention that if you are required to take three or more strips, wait until the first two dissolve on each side of the mouth and then place the third strip in your mouth and repeat the process.

This means that the film strips and the dissolved Suboxone residue could be in your mouth for 1 hour or more, exposing your teeth to an extremely acidic environment.
An extreme but fair analogy would be something like taking a sip of vinegar and then holding it in your mouth for 30-60 minutes. If you try this—or if you take Suboxone—rinse your mouth out afterward so that you can remove the acid and return your mouth to its regular pH.
After reading this particular section, consider how many Suboxone users were unaware of any of this because it was not told to them or their doctors. Think how many people went to bed with a Suboxone strip in their mouth or tooth their medicine three times a day for years and never rinsed afterward because they didn’t know any better. This is where the failure to warn accusations against Indivior really hit home.
Spotting Early Signs of Tooth Decay from Suboxone
If you’re using Suboxone, it’s especially important to pay close attention to your mouth’s health and to know how to spot early signs of tooth decay. Here’s what to look for:
- Increased Sensitivity: If your teeth suddenly find hot or cold temperatures or sweet flavors painful, it could be an early warning sign that your enamel is breaking down or that you already have a cavity.
- Toothache or Sharp Pain: Unexplained or sporadic pain can signal decay as bacteria enter your tooth’s nerves. As we have discussed, Suboxone may be feeding bad bacteria into your mouth, so this is an important indicator.
- Noticeable Holes or Pits: Any visible marks or holes in one of your teeth or something that feels odd with your tongue could be a sign that decay has started.
- Stains on Your Teeth: Look out for unusual staining, mainly dark brown or black spots or oddly white spots, as these can indicate the presence of decay. Even though there is not a cavity or hole in your tooth yet, it doesn’t mean you don’t have decay. Catching it at this stage is better than the alternative.
- Dry Mouth: Suboxone can cause reduced saliva production in your mouth, leading to dry mouth. This can be dry mouth like a desert, but it can also just be a large decrease in saliva, but still not enough for you to really notice. This makes it all the more problematic. Saliva is essential for washing away food in your mouth and neutralizing acids produced by bacteria, so a lack of it can speed up decay by allowing those bacteria feasts to stick around.
- Bad Breath or Unpleasant Taste: Persistent bad breath or a bad taste in your mouth can be a sign of decaying teeth and accumulating bacteria. Make sure that you brush your tongue and floss, as these are usually the cause of bad breath, precisely because these areas are holding old food and bacteria, which are rotting and starting to stink. Kind of makes you want to brush your teeth, right?
Taking extra good care of your teeth while you are on Suboxone, rinsing out your mouth after you take your medicine, and knowing the early signs of tooth decay can all make a big difference in preventing the acidic medicine from doing real damage.
Again, this is why it is so important to be educated about these issues when you start taking a new medication. Would you have denied Suboxone if you had known it might cause tooth decay? Probably not. But you likely would have taken the necessary steps to guard your oral health had you been warned about the potential side effects.
Suboxone Tooth Decay Lawsuit Eligibility
Suboxone tooth decay is a harmful symptom that has negatively impacted many, but not all are eligible to file a Suboxone tooth decay lawsuit.
Only those who used Suboxone before 2022, when there was no warning of tooth decay in the labeling, will be able to participate in Suboxone litigation. The new warnings provided on the Suboxone label show that there is real truth to these claims and demonstrate how important regular dental checkups truly are.
Furthermore, prospective plaintiffs need to be able to prove their use of Suboxone and must have suffered tooth decay injury severe enough to warrant filing a lawsuit.
Filing a Suboxone lawsuit is not something to take lightly, and the damages suffered by a plaintiff need to necessitate the investment of time and effort that goes into filing a lawsuit like this.
Who Qualifies for a Suboxone Lawsuit?
To qualify, you must meet all of the following requirements:
- You were prescribed Suboxone by a doctor
- You used Suboxone for at least 6 months before 2022
- You are suffering from cavities, tooth loss, tooth fractures, tooth decay, tongue injuries, and gum injuries that you did not have before taking Suboxone
- You had routine dental care before starting Suboxone to prove the prior condition of your teeth
If you are questioning whether or not your injuries entitle you to seek compensation from Indivior in a Suboxone tooth decay lawsuit, contact Dolman Law Group for a free consultation where our Suboxone tooth decay lawyers can answer your questions and determine your eligibility.
Preserving Evidence for Your Suboxone Tooth Decay Case
If you have severe tooth decay because of taking Suboxone, you need to preserve evidence so that your lawyer can build a strong case for you. Here’s what you can do to make sure you have the best chance possible at winning your case and getting compensation:
Gather Your Suboxone Prescriptions
Try to find any of your Suboxone prescriptions that you can and keep all your future prescriptions. You can also contact your doctor and pharmacist to see if they can help you get copies. Documentation of your Suboxone prescriptions shows your dosage and changes in dosage, how long you’ve been on Suboxone, and other details.
Maintain Dental Records
Don’t forget to continue your regular dental checkups and keep all your records. You should also try to get your past dental records, including X-rays, diagnoses, and treatment plans related to your tooth decay. These documents will show the extent of your dental damage and help provide a timeline of your issues. And, if you’ve had any major procedures because of your dental decay, keep the receipts and any related paperwork.
Document Doctor Records
If you ever talked to your doctor or dentist about how Suboxone is impacting your teeth, keep copies of any paperwork or notes from those conversations. You can also get copies from your doctors or dentists. Not only can this help provide context for your condition, but if you brought up any issues, it can also potentially show documentation if you raised any concerns and weren’t informed about the risk of Suboxone causing dental problems.
Take Photos
You should also take clear photos of the damage to your teeth. Any photos will be helpful, but if you can get pictures as your dental decay progresses. This will help the jury or defendants see the extent of your decay and understand the impact on your life.
Keep Receipts for Dental Expenses
Keep receipts of what you paid for dental procedures and anything else related to costs that show how much you spent treating your tooth decay. These will be used to document the financial burden you’ve experienced.
Maintain a Journal
If you realize what is happening soon enough, write about it in a journal or keep notes about your experience on your phone. Keep track of the dates you started and stopped taking Suboxone, any symptoms you experience related to your teeth (like dry mouth, increased sensitivity, cavities, etc.), and anything you talked to your doctor about. Really, this step, and all the others, is about gathering as much evidence as possible to show your negative experience with Subxonee and tooth decay.
Speak to a Lawyer
Don’t be discouraged if you’re missing some of the above evidence. A Suboxone lawyer can still offer valuable guidance, help to prove your case, and use expert witnesses to prove how Suboxone caused your tooth decay.
Talking to a lawyer about your case is one of the most important things you can do to ensure you get compensation for your tooth decay. This is different from the kind of lawsuit you can handle alone. Matt Dolman, Stan Gipe, and all of Dolman Law Group have been taking on giant corporations and pharmaceutical companies for decades. We have the expertise and resources to take on Suboxone and Indivior, precisely what we are doing.

Damages in a Suboxone Lawsuit
Patients who have experienced severe tooth decay and other mouth-related problems caused by Suboxone may be able to recover compensation for their losses. The amount they can recover depends on the severity of the dental damage, as illustrated by prior dental records, the specific details of the case, and the results of the court proceedings.
The losses caused by Suboxone-inflicted tooth decay are called damages and are what these lawsuits seek to compensate either through a settlement or court awards. These damages may not only involve economic damages with direct dollar values but also encompass non-economic damages such as mental anguish that are abstract and intangible losses that are still considered.
Potential damages in a Suboxone tooth decay lawsuit might include:
Medical Expenses
Victims may be eligible to receive compensation for medical bills for dental treatments like fillings, root canals, extractions, and other necessary procedures. Medical expenses covered by a Suboxone settlement can also include prescriptions, medical devices, and further doctor appointments.
Pain and Suffering
Compensation may also cover physical and emotional pain and suffering endured as a result of dental issues caused by Suboxone.
Lost Wages
If the dental problems resulted in missed work or a loss of income, individuals may be able to recover that money by filing a claim. Lost opportunity for promotion, loss of a job position, or loss of income opportunity in general can also be considered for compensation in a Suboxone settlement.
Future Medical Costs
In cases where ongoing dental treatment is needed, compensation can include future medical expenses related to dental care and other issues caused by Suboxone.
Punitive Damages
In some cases, if it can be proven that the manufacturer’s actions were especially reckless or negligent, punitive damages may be awarded to punish the responsible party and deter similar behavior in the future.
How Dolman Law Group Can Lead Your Tooth Decay Suboxone Case
Dolman Law Group specializes in pharmaceutical litigation and personal injury cases involving dangerous defective drugs. We understand the complexities of Suboxone tooth decay lawsuits and can provide expert legal guidance.
The product liability lawyers of Dolman Law Group are well known for taking on large drug manufacturers. Pharmaceutical companies involved in past cases were larger than Indivior, Inc. We have filed lawsuits against the biggest drug manufacturers behind medications like Zantac, Tylenol, Uloric, Tepezza, Ozempic, and more.
Our Suboxone tooth decay lawyers will thoroughly evaluate your case and review your medical records, dental history, and the circumstances of your Suboxone use so we can determine its strength and whether it’s worth pursuing. We have compiled substantial research on how Suboxone films impact your long-term dental health.
Learn Whether You Qualify for a Suboxone Dental Lawsuit
Seeking a Suboxone tooth decay lawyer is paramount if you have suffered dental and oral damage as a result of using Suboxone films before 2022, since Suboxone’s label failed to warn of this symptom adequately.
With the help of our experienced lawyers, your Suboxone tooth decay case will be prepared to obtain more than fair compensation for your damages. We will fight for the maximum value available through a Suboxone settlement or court award.
Our Suboxone lawsuit lawyers are ready to help you receive the compensation you need for your dental problem caused by Suboxone. We are handling Suboxone lawsuits on a national basis.
If you believe you have a case, call our Suboxone lawyers at 727-451-6900 or contact us online. Let us help you determine whether you qualify to file a Suboxone lawsuit. If your doctor prescribed Suboxone and you suffered severe dental injuries, we want to hear from you today.
Non-website related resource:
[1] Berkson, L., & Montero, A. M. (2016). Suboxone and dental caries: A case-control study. Journal of Clinical Pharmacy and Therapeutics, 41(11), 938-942.
[2} Etminan, M. & Rezaeianzadhe, R. (2022) Association Between Sublingual Buprenorphine-Naloxone Exposure and Dental Disease: Journal of the American Medical Association, 328(22): 2269–2271
[3] Buprenorphine: Drug Safety Communication – FDA warns about dental problems with buprenorphine medicines dissolved in the mouth to treat opioid use disorder and pain, (2022) U.S. Food & Drug Administration
