Lawsuits against Bard Access Systems, Inc., and its parent company, Becton, Dickinson & Co., are being filed for failure to inform and warn patients and healthcare professionals about the potential risks associated with the Bard Power Port.
The nationwide Bard Power Port Lawsuit involves patients who have reported dangerous conditions, such as blood clots and serious infections, after receiving a Bard Power Port implant.
Patients who have been injured by the Bard PowerPort—which is widely used in chemotherapy treatments—are pursuing financial compensation for the damages they have suffered, including medical expenses, lost income, and physical and emotional distress.
Bard PowerPort Lawsuit Updates
At Dolman Law Group, we have decades of experience helping injured people recover compensation from responsible companies that manufacture and distribute dangerous medical devices. We began monitoring the Bard PowerPort news before the first lawsuit was filed, and the federal cases were consolidated into a Mutli-District Litigation (MDL).
Bookmark this page and return often for the latest updates regarding the Bard PowerPort Lawsuit progress.
Court Order Addresses Discovery Deadlines, Strongest Claims Defined
January 1, 2025 – The December status hearing brought about a new Case Management Order that extended the fact discovery deadline to the end of February 2025, with expert discovery continuing through July. The MDL court also emphasized the importance of both parties adhering to previous orders and resolving any outstanding issues related to fact sheets before the end of the year.
The court will not schedule bellwether trials—test cases meant to guide the direction of the litigation—until the discovery phase is completed. This means that settlement progress is unlikely in the near term. Without the imminent pressure of a scheduled trial, the defendants are not expected to make any reasonable offers.
The strongest claims in this litigation involve patients who received the Bard PowerPort catheter within the past decade and subsequently experienced severe complications. These include issues such as device leakage, breakage, or migration, where the implant moves to unintended areas of the body. These complications have led to serious consequences, such as infections, blood clots, and critical heart conditions. In some cases, patients have required surgical removal of the device, facing further complications like infections, hemorrhaging, organ perforation, tissue lacerations, and necrosis (tissue death).
Many of the claims allege that the PowerPort manufacturers failed to adequately warn about these risks. Plaintiffs also argue that the device underwent insufficient testing and that key information was not disclosed, leading to claims of strict liability and fraudulent misrepresentation. Additionally, several plaintiffs are seeking punitive damages to hold Bard and its successor company accountable for these issues.
Bard MDL Reaches 720 Lawsuits, All Bard-Related Companies May be Liable for Injuries, No Discovery Extension Granted
December 1, 2024 – The Bard Power Port MDL has expanded to include 200 additional claims, bringing the total number of cases to 720. The court has also directed certain plaintiffs to comply with prior case management orders, warning that failure to do so could result in their lawsuits being dismissed.
A significant liability issue has also been resolved. The parties have agreed that all Bard-related companies, including Becton, Dickinson, and Company, which acquired Bard during the period when these claims arose, will collectively bear responsibility for settlements or judgments. This stipulation broadens the pool of financial resources available to plaintiffs.
In other updates, the court maintained its stance on the current discovery deadlines, declining the plaintiffs’ request for an extension. However, the court did authorize a critical deposition and is evaluating whether plaintiffs may gain access to certain privileged documents. Additional developments on this issue are expected soon.
Plaintiffs Fear They May Need More Time to Prepare For Trial
November 1, 2024 – The current scheduling order includes deadlines for evidence discovery, expert disclosures, and motion hearings. The lawyers for the plaintiffs have requested more time to ensure they are properly prepared, but the defense won’t agree to the proposed extensions.
Under the pending order, all discovery must be done and motions filed by September 8, 2025. Plaintiffs’ counsel wants to push that date back to October 13, 2025. We’ll let you know when the court rules on this issue.
Successor Liability is Hotly Disputed in This MDL
October 1, 2024 – Defective product mass tort cases are usually based on the product manufacturer’s negligence in creating a dangerous product or failing to warn about possible harm. C.R. Bard, Inc. manufactured the Bard PowerPort device, but in 2017, Bard was purchased by a successor company, Becton Dickinson.
The legal theory of successor liability allows an injured person to request compensation from the original company involved and any company that acquires a financial interest in the original manufacturer. The plaintiffs in this MDL want both companies to be held responsible for their injuries and pay for the damages caused by the alleged defective PowerPorts. To avoid responsibility, the defendants are refusing to provide information that might explain the corporate relationship. So far, the court has not agreed with the defendants’ arguments.
While C.R. Bard is a large corporation, Becton Dickinson is larger, showing an estimated market cap value of over $65 billion. Our team expects the additional insurance coverage and financial resources will affect settlement discussions and improve the chances that plaintiffs will receive more compensation if both companies are found liable.
Recent Claims Allege Bard Power Ports Lead to Infection and Thrombosis
September 1, 2024 – Two of the most recent plaintiffs joining the Bard multidistrict litigation have alleged serious medical conditions related to the failure of their Power Ports. A man from Memphis claims he received a power port in 2018 that later caused serious infection and vein thrombosis. Also, a woman in Missouri alleged she received a power port in 2012 that led to thrombosis and severe medical issues.
If you or someone you love received a Bard Power Port and then developed problems when it fractured, migrated, or caused infection, you may be eligible to bring a lawsuit against the manufacturer and other responsible entities. Reach out to the experienced mass tort team at Dolman Law Group to learn more.
August Case Management Conference Addresses Several Important Matters
August 16, 2024 – The 9th Case Management Conference was held today in the Bard PowerPort MDL. The court focused its attention on several issues. First, four plaintiffs have not completed a Plaintiff Profile Form as required by the court. They have one week to finalize that filing. The court also placed a three-hour cap on certain witness depositions.
Another important pending issue is whether Bard’s parent company, Becton Dickinson (BD) can be held liable for Bard’s manufacturing and marketing of the alleged defective medical ports. Under the theory of successor liability, a company that acquires another company can be found responsible for the acquired company’s liabilities. Plaintiffs are considering five theories of successor liability to keep BD involved in this litigation.
Bard MDL Momentum Slows Over the Summer Months
July 31, 2024 – As of the end of July, 322 Bard PowerPort claims have joined the pending MDL. However, beyond scheduling another regular status conference, no further progress has been made toward setting dates for the test trials, known as bellwether trials, or encouraging settlement. The next court date is August 16, 2024, so we hope to see the litigation start moving again.
Judge Requires Joint Memorandum in Bard PowerPort MDL
July 2, 2024 – The Bard PowerPort multidistrict litigation (MDL) has reached a significant milestone, with roughly 299 lawsuits currently active under Judge David G. Campbell’s oversight in the District of Arizona.
This consolidation brings together cases nationwide, all centered on allegations of severe health complications arising from purported design defects in Bard’s PowerPort devices.
Plaintiffs in these cases report a range of serious medical issues, including device fractures, infections, and blood clots, among other critical injuries.
As the MDL progresses, it illuminates the potential risks of these medical devices and their broader implications for patient safety in the healthcare industry.
Both Sides Agree to Electronic Disclosures and Deadlines
June 6, 2024 – In a Multidistrict Litigation (MDL) case, a joint memorandum is a document prepared by both plaintiffs’ and defendants’ attorneys to address specific issues the judge wants clarified. This helps streamline the pretrial process by saving time and resources.
The joint memorandum outlines areas of agreement and disagreement between both parties, allowing the judge to focus on unresolved issues. For the upcoming joint memorandum due on July 8, 2024, the parties have agreed on search terms for electronic information for the first 30 custodians, with a deadline for completion by July 1, 2024. Discussions are still ongoing for the second group of 30 custodians, and no agreement has been reached on the turnover date for important documents from DocuShare. The memorandum will also address efforts to agree on successor liability involving Bard and Becton, Dickinson and Company (BD).
Resolving these discovery disputes is essential for proceeding to bellwether trials, which involve a jury trial to test legal theories and evidence.
New Power Port Injury Alleged, Bard MDL Adds 50 New Lawsuits
May 2, 2024 – A Texas woman filed a lawsuit against Bard, alleging her implanted Power Port was defective and led to atrial fibrillation. This alleged injury is different than the rest of the catheter cases we have seen that focus on internal infections, broken and migrating catheters, and thrombosis.
With the addition of nearly 50 new claims last month, the MDL now has 187 pending lawsuits and still growing.
Judge Focuses on Bellwether Trial Selection, Discovery, Evidence Preservation Process, and Whether Former Bard CEO Should Be a Document Custodian
April 10, 2024 – Since our last update, Judge Campbell issued a flurry of case management orders to keep this MDL moving quickly. Plaintiffs are allowed to amend the Master Complaint to include port reservoir failure allegations, and all plaintiffs must use the Short-Form Complaint or risk having their cases dismissed.
A biomaterial preservation order now describes detailed protocols for handling, storing, testing, and examining explanted devices and other physical evidence. Both sides are allowed non-destructive evaluations and additional testing as needed. The goal is to document the evidence chain of custody and ensure both sides have equal access to relevant evidence.
Lastly, plaintiffs have suggested naming Tim Ring, Bard’s former CEO, as a document custodian so they can request and examine his records. Of course, Bard is objecting in order to protect its upper management. This issue can play a pivotal role in complex litigation because it may show what the company knew internally and how truthful its public statements were. The number of Bard PowerPort lawsuits in this MDL is now 154.
Two Bard Power Port MDL Plaintiffs Have Died. Number of Pending Cases Reaches 106
March 4, 2024 – The Bard Power Port MDL recently received two Suggestions of Death. This happens when a plaintiff dies, and their personal injury lawsuit is still pending. A Suggestion of Death is a formal notification to the court that the party has passed away. The official plaintiff must be changed to the legal representative handling the plaintiff’s estate. Additional counts may also be added to the complaint to request damages for the plaintiff’s wrongful death and a survival action for the remaining family members.
In the past few weeks, we received notice that two of the original Bard Power Port MDL plaintiffs passed away. These deaths show how severe these injuries are and why injured plaintiffs need financial justice as soon as possible. By the end of February, seven more lawsuits joined the Bard Power Port MDL. The total number of cases is now 106.
Second Flaw Found in Bard PowerPorts May Add New Plaintiffs to the MDL
January 17, 2024 – The Bard PowerPort multidistrict litigation has grown to include 73 claims, which deal with the fracturing and dislocation of the defective polyurethane catheter tubing. Plaintiffs assert that Bard manufactured weak tubing because they used an excessively high concentration of barium sulfate to the point where it was not dispersed evenly throughout the product. These Bard PowerPort users are seeking considerable damages after their broken implants caused them to develop blood clots, infections, and other serious injuries.
Bard was aware of these risks and did not correct the design or warn patients and their providers. The other group of plaintiffs has leveled similar accusations of negligence against Bard Access Systems and its parent company. However, they sustained their injuries due to the deterioration of the implant’s port reservoir component, which relied on a questionable material known as polyoxymethylene to construct the port. The Judicial Panel on Multidistrict Litigation is set to address the topic of consolidating the new Bard PowerPort claims at the end of January.
New Bard PowerPort Infection Lawsuit Filed in MDL
January 9, 2024 – A Mesa, Arizona man recently joined the Bard PowerPort MDL, alleging he suffered a serious infection as a result of a PowerPort isp M.R.I. implantable port. He attributes his infection and related financial losses to port defects, including manufacturing flaws. The lawsuit is brought against Bard Access Systems, Inc., Becton, Dickinson and Co., C.R. Bard, Inc., and Bard Peripheral Vascular, Inc.
Attorneys Receive More Time to Submit a Joint Order Addressing Preservation of Evidence
January 5, 2024 – Requests to preserve evidence are an important tool that allows parties to ensure that relevant evidence is protected and disclosed. This evidence can include everything from internal memos and emails to device blueprints or financial statements. Plaintiffs will be looking for proof that Bard Access Systems failed to warn the medical community and the public at large about the increased risk of complications such as blood clots caused by their flawed port-catheter devices.
Judge Campbell has granted additional time to file a joint order on this topic, given the scope and gravity of the issue. A status conference is scheduled for January 8, 2024. In the past two months since the last status conference, fewer than a dozen Bard PowerPort lawsuits have been added to the MDL.
71 Bard PowerPort Lawsuits Consolidated into MDL in 2023
December 26, 2023 – The Bard PowerPort MDL, located in the U.S. District Court in Arizona and overseen by Judge David G. Campbell, now includes 71 lawsuits. These cases, originally filed in various federal courts across the country, were consolidated into this MDL in September. The claims in these lawsuits are centered around similar complications experienced by plaintiffs due to the implanted port catheter system. The issues cited involve the degradation of barium sulfate particles from the Bard PowerPort, leading to device malfunctions or migration and, in some instances, particles entering the bloodstream, causing various severe health complications.
MDL Judge Enters Case Management Orders Regarding Discovery and Bellwether Trial Schedules
December 3, 2023 – Judge Campbell has issued several case management orders detailing the discovery process and timelines leading up to bellwether trials. These trials serve as test cases to help both sides assess jury reactions to the scientific evidence presented. The outcomes of these trials will guide potential settlements and resolutions of the cases. The discovery phase is scheduled from November 20, 2023, to January 31, 2025. Both sides will then submit expert reports, and the trials will likely start in mid-2025.
Bard PowerPort Lawsuit Master Complaint Approved
November 16, 2023 – The presiding MDL Judge has approved a master complaint form. Now plaintiffs can file their Bard PowerPort catheter lawsuits directly into the MDL without having to file in another federal court and request a transfer to the MDL.
Judge Campbell Requests Joint Order Regarding Protecting and Preserving Evidence
October 17, 2023 – Judge Campbell requested that the plaintiffs’ and defense’s counsel submit protective and preservation orders by October 27, 2023. He also instructed both sides to agree on a short-form complaint, facilitating direct filings of Bard PowerPort lawsuits into the MDL.
Appointment of Leadership in Bard PowerPort Litigation
September 19, 2023 – Judge Campbell appointed a leadership team for the port catheter MDL, including 39 lawyers on the plaintiff’s steering committee for the Bard Implanted Port Catheter Products Liability Litigation. The team includes three lead attorneys, eleven attorneys as part of the Plaintiff’s Executive Counsel (PEC), and twenty-five additional lawyers on the Plaintiff’s Steering Committee (PSC) and its sub-committees.
JPML Creates an MDL for All Federal Bard PowerPort Lawsuits
August 8, 2023 – The Judicial Panel on Multidistrict Litigation (JPML) centralized all Bard port catheter lawsuits from federal courts nationwide into the U.S. District Court for the District of Arizona. The MDL was assigned to Judge David G. Campbell and titled the Bard Implanted Port Catheter Products Liability Litigation. We believe Judge Campbell was chosen because he has prior MDL experience from overseeing the Bard IVC filter MDL.
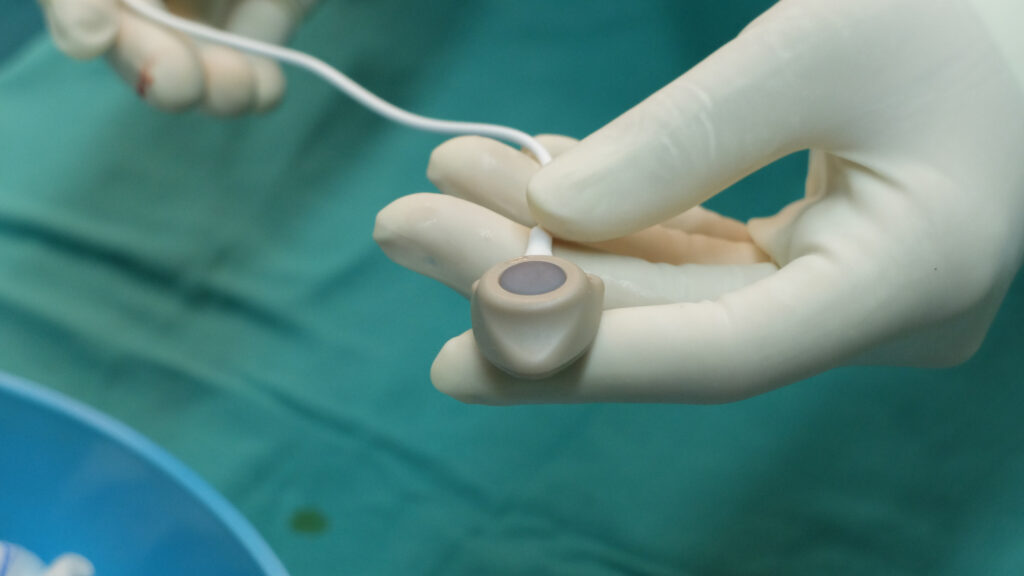
How Bard PowerPort Catheters Are Used

To help administer medications, chemotherapy, or other fluids, doctors may implant the Bard PowerPort under the skin, usually into the chest or an arm. This catheter device has a port with one or two sealed basins for accepting an IV or injection.
From the port, catheter tubing enters into a central vein to allow the fluid to be pumped directly into the bloodstream. This method improves the way your system accepts the medication. The majority of the pending Bard PowerPort product liability lawsuits raise the legal theory that the catheter tubing is flawed because it can break off and cause pieces to enter the body, or the tubing can become pitted, which can lead to infection.
The primary goal of a power port is to allow a healthcare provider to use a Huber needle to access the port while causing minimal pain for the patient. Huber needles are non-coring needles, and because of their smaller diameter, they don’t cause as much pain for a patient who requires repeated access for medical providers to administer antibiotics or chemotherapy medications or withdraw blood on a regular basis. Without an implated port, patients must endure frequent large needle sticks, increasing their pain and discomfort.
These Bard PowerPorts also allow fluids to enter the patient’s system at a faster rate. Because of this power injection capability, ports can also be used when the patient needs a Contrast-Enhanced Computed Tomography (CECT) scan. In these CT scans, contrasting fluid, which acts like a dye, is injected through the port to create a distinction during the scan, which allows providers a better view of problematic areas.
The Scientific Basis Explaining How Bard PowerPorts May Be Defective
Research is looking into a potentially dangerous design flaw in the Bard PowerPort polyurethane catheter tubing. To be curved into a blood vessel, this tubing must be flexible. However, it appears the combination of materials needed to make the tubing pliable damages its structural integrity. The catheter tubing created by Bard contains barium sulfate, which creates contrast during X-rays, along with polyurethane, which is a type of polymer most often used in the production of various foam products.
If these two materials are improperly mixed or combined, the barium sulfate can break down the polyurethane portion of the catheter. As the catheter fails, it may crack, causing parts of the tube to enter other parts of the body. Also, some patients have reported infections involving the power port that may have developed from small pits in the tube that collect and hold bacteria. In their lawsuits, plaintiffs have alleged the Bard PowerPorts contain too much barium sulfate and the catheter failed as a result.
This argument claims the concentration of barium sulfate was too dense to blend with the polyurethane, such that it was not evenly distributed. It is believed the design defect causes small pockets that damage the tubing and make the Bard PowerPort tubing more brittle not flexible, leading to breakage and further medical complications.
What Injuries Are Reported By Patients With Defective Bard PowerPorts?
Patients are reporting a variety of injuries and medical problems after receiving defective Bard PowerPort catheter implants. These problems can be serious, and some are even life-threatening. The injuries are allegedly related to decomposing tubing and parts of the device breaking away and ending up in different parts of the body where they must be surgically removed.
The dangerous device and product liability lawyers at Dolman Law Group are working with many clients whose power port devices fractured, failed, and led to broken pieces floating around their bodies. We believe these medical concerns are related to Bard’s defective design and that Bard should be held responsible for our clients’ damages.
Throughout the nation, many patients report cardiovascular damage after receiving these flawed ports. One of the main concerns related to these implantable devices is a greater risk of blood clots because clots can travel to important organs such as the heart, lungs, or brain and cause heart attacks, strokes, or other serious issues. At this point, patients have experienced several serious medical problems after receiving Bard PowerPorts, including:
- Bacterial Infections
- Deep Vein Thrombosis, frequently referred to as DVT
- Vascular Damage
- Cardiac arrhythmia, also known as an irregular heartbeat
- Cardiac Punctures
- Cardiac or Pericardial tamponade, pressure on the heart caused by too much fluid or blood
- Organ Damage
- Heart attack or myocardial infarction
- Hemorrhages, a substantial blood loss from a damaged vessel
- Hemothorax, or the pooling of blood between the lungs and chest wall
- Pneumothorax, known as a collapsed lung
- Hematomas
- Pulmonary embolism
- Pulmonary pseudoaneurysms, injured blood vessels in the heart
- Stroke
- Tachycardia, an accelerated heartbeat
Patients Who Receive Bard PowerPorts Face Extra Complications Because They Already Have Existing Health Problems
Most of the patients who receive Bard PowerPort catheters need them to receive medical care for serious conditions such as cancer. If a catheter tube malfunctions, these already weakened patients cannot fight a new problem or infection, and they are more likely to suffer greater damage as a result. For example, cancer patients who need a power port to receive chemotherapy treatments usually have weakened immune systems, so if a defective PowerPort causes an infection, their body can’t fight back, and it could lead to a life-threatening situation.
If you suspect a Bard PowerPort has created additional medical problems, or if you or someone you love are injured by a catheter device, speak with a knowledgeable medical provider immediately. To learn more about your potential legal rights if a defective Bard PowerPort has harmed you, reach out to the compassionate and tenacious personal injury lawyers at Dolman Law Group as soon as possible.
Crucial Tip: Always seek medical care as soon as possible if you think a defective medical device has harmed you. There are two important reasons to consider. First, complications from defective medical devices can be extremely serious and may place your life or recovery in danger without proper medical treatment.
Second, if you wait to be treated, any delay might harm you if you pursue a personal injury claim. Insurance companies often try to challenge patients’ credibility or reduce settlement offers by arguing the condition is not as serious as claimed. Also, without medical records and expert medical testimony to support a claim against a medical device maker, an insurance company may offer a lowball settlement or try to deny the claim entirely.
How Bard Buried Evidence About Critical PowerPort Issues
Plaintiffs who have filed Bard PowerPort lawsuits have claimed the manufacturer intentionally withheld crucial information it knew about the defective catheter tubing used in the PowerPorts. Specifically, when medical providers discovered their patients were experiencing blood clots and infection problems after the device was implanted, they connected the issues to the deteriorating catheter tubing. Many of these professionals reported these adverse events to Bard Access Systems and its parent company, Becton Dickinson & Company.
In fact, these adverse events have been repeatedly reported to Bard Access Systems ever since Bard PowerPorts became available for public applications more than 20 years ago. Also, similar port catheter product users have reported considerably fewer complaints, which reinforces the legal theory that Bard knew or should have reasonably known that its PowerPort catheter tubing was dangerous. Yet, Bard never warned anyone about these potential dangers.
Did Bard Evade Accountability for Decades?
After physicians notified Bard of potentially harmful defects, it appears Bard failed to mention any device problems. Bard also received special clearance from the FDA to market its PowerPort catheter devices without the normal level of testing required for other medical devices.
The Bard PowerPort actually received early FDA approval (without the usual level of testing) under the agency’s §510(k) provision because Bard’s device was considered similar enough to other port devices that had already received FDA approval. Bard was basically allowed to circumvent standard FDA testing and reporting protocols that likely would have required public disclosure of the adverse event reports.
Instead of taking responsibility and issuing a recall or a warning, Bard Access Systems and its parent company did not warn medical providers or patients while continuing to produce and distribute defective PowerPorts. It is also believed that Bard inaccurately claimed that the problems associated with the catheter tubing were caused by surgical error. We expect this conduct will likely be used against Bard to prove liability in the personal injury lawsuits filed against this negligent company.
Bard PowerPort Lawsuits Allege Failure to Warn and Defective Design Issues
To succeed in a product liability lawsuit and receive compensation for related damages, a plaintiff must prove additional requirements beyond those required in an average personal injury lawsuit. The alleged injury must be caused by a harmful defect and must be linked to:
- A design problem
- Improper manufacturing practices
- Careless or negligent product distribution or
- The manufacturer failed to warn the public about the risks and dangers associated with its product
In the Bard PowerPort claims, we believe injured plaintiffs might argue several legal theories to hold Bard Access Systems liable for the injuries caused by its product.
A Bard PowerPort Lawsuit Might Be Based on Defective Product Arguments
As mentioned above, since the polyurethane catheter tubing was poorly designed and the ratio of the two main ingredients was incorrect, these two errors could render the product defective. In addition, the manufacturing process did not address these errors, which means the catheter tubing was inclined to break down. Finally, Bard Access Systems and its parent company did not warn the medical community or patients about the known problems and potential dangers associated with their PowerPorts.
Companies that produce medical devices have a legal responsibility to warn prescribers and users about dangers the company knew or should have known about. Specifically, Bard had the legal obligation to warn about the potential trouble their polyurethane catheter tubing could cause,
When the maker of a faulty product fails to meet their duty of care to consumers by creating a dangerous product and concealing harmful defects, they can be held liable and ordered to compensate injured users for the damages they experienced after being harmed by the defective medical device. Under product liability theories of law, injured plaintiffs are protecting their rights to seek compensation in the Bard PowerPort multidistrict litigation.
Which Damages Are Available in a Bard PowerPort Lawsuit?
Because the potential injuries involved in a product liability claim related to implanted catheters can be life-threatening, the damages these patients face can be extensive. Patients injured by Bard PowerPorts may suffer extensive medical costs caused by defective catheter tubing, including serious infections, blood clots, organ damage, and permanent disability caused by a stroke.
In general, product liability lawsuits allow an injured party to seek compensation for both economic and non-economic damages. The legal theory is an injured person has the right to compensation that will return them to the same position they were in before they were injured by a defective medical device. However, that usually can’t happen. Instead, our goal at Dolman Law Group is to fight for the maximum amount of compensation possible to help our clients move forward and have the best life possible after suffering an injury caused by someone else’s negligence.
The Most Common Damages Suffered by Implanted Port Patients
If your Bard catheter tubing splintered or parts of your port moved from the original implant location, you are likely facing bills for additional medical treatment to repair the damage or remove the broken pieces. You’ve probably missed work because you needed extra medical care or time off to recover, and you may be dealing with other unexpected expenses.
We know these financial costs can add up, but we also know how to demand payment on your behalf in the form of economic damages. We also understand how intangible injuries, like stress and pain, can affect your life, and we’ll fight to recover these non-economic damages for you as well. Injured plaintiffs in a Bard PowerPort lawsuit may recover payment for:
- Medical bills
- Lost wages
- The cost of removing a failed port and implanting a new port
- Pain and suffering
- Loss of quality of life
- Wrongful death if the failed port caused someone’s death
Who is Eligible to File a Bard PowerPort Lawsuit?
If you or a loved one has used a Bard PowerPort now or in the past, you may wonder whether you can add your name to the list and file a lawsuit against Bard Access Systems. The best way to determine whether or not you’re eligible is to speak with an experienced Bard PowerPort lawyer about your potential case.
However, here are a few key criteria that can help determine whether or not you’re eligible to file a lawsuit against Bard Access Systems for their failure to warn users of potentially dangerous side effects.
- You have documented injuries or complications as a result of having a Bard PowerPort
- A loved one who had a Bard PowerPort device implanted passed away because of suspected complications with the device
- You required additional medical intervention to remove the PowerPort device or to treat complications
- You have experienced additional financial losses as a result of the device
If you’re uncertain whether or not your medical complications are connected to your PowerPort device, it’s important to seek medical attention and talk to your doctor. Ongoing medical treatment is as important as working with an experienced lawyer throughout the process!
How to Prove that Your Bard PowerPort Caused Serious Medical Conditions
In order to receive compensation for your damages, you must be able to prove that your Bard PowerPort directly caused medical complications such as blood clots, heart attack, stroke, or other issues. However, while your personal medical evidence is vital to the case, other information, such as studies performed on the devices themselves, medical expert testimonies, and more, will be vital to proving that Bard Access Systems acted negligently in their creation and marketing of the device.
In combination with evidence from various cases, we are confident that we’ll be able to prove the link between Bard PowerPort catheters and medical complications such as blood clots. We are passionate about ensuring that Bard Access Systems pays out large settlements to the customers they have wronged.
Why You Should Have a Bard PowerPort Lawyer Fighting For You
If you have a Bard PowerPort, you were facing serious medical issues before you received the catheter port. The personal injury lawyers at Dolman Law Group work with many people who were already dealing with serious health issues like cancer before a defective medical device worsened their condition. If you experienced an additional injury because of a defective PowerPort, you shouldn’t have to expend the time or energy needed to effectively represent yourself in a product liability claim.
Dolman Law Group’s legal team offers the experience, skill, and resources you need to successfully navigate the personal injury claims process. We can bring you peace of mind during your complicated situation and allow you to focus on your physical and mental recovery while we handle the legal challenges.
Like all businesses, medical device companies are in business to make a profit and protect their reputation. Accepting fault in a product liability lawsuit does not further those goals. To protect their financial interests, companies like Bard Access Systems and Becton Dickinson & Company, along with their insurance companies, keep an aggressive legal team on salary. You’ll need a fierce team of personal injury lawyers in your corner fighting back to ensure you receive the compensation you deserve.
Hiring an award-winning product liability attorney to represent your case proves to the defendant’s legal team that you are willing to fight for a settlement or verdict to cover your specific losses. When we work together, we typically won’t accept the first offer just to resolve the case quickly. We are ready and willing to fight to protect your rights and not back down against big corporations and insurance carriers.
The Product Liability Lawyers at Dolman Law Group Offer Critical Legal Assistance to Injured Plaintiffs
Our personal injury lawyers have extensive experience negotiating defective medical device claims, unlike many other law firms. Using our decades of skill, our goal is to ensure your claim is protected and fairly compensated. Our team has successfully represented clients seeking payment for medical device injuries like metal-on-metal hip implants and CPAP breathing machines, and we are not afraid to take on big medical device makers like Bard Access Systems.
When you partner with our defective medical device lawyers, we will work tirelessly to build a solid claim for the compensation you need by showing how the defendant was negligent, proving that they are liable for your losses, documenting your financial damages, and strategically advocating for the best possible settlement or verdict.
Contact the Experienced Bard Powerport Lawyers at Dolman Law Group to Discuss Your Bard PowerPort Lawsuit
Patients should be able to trust that the medical devices used in their care are safe and effective, but we know this is not always possible. The Bard Powerport catheter has harmed the health and finances of numerous vulnerable patients, and the manufacturer should be held responsible for failing to correct the defects or warn the public about the risks.
When a company like Bard Access Systems prioritizes profits over patient safety, it should be held accountable for the harm it created. Dolman Law Group’s team of accomplished medical device lawyers will gather the evidence needed to show that Bard’s negligence led to life-altering injuries that unfairly harmed already sick patients and caused additional medical bills, lost wages, pain and suffering, and other damages, including emotional distress.
We are following the Bard Powerport multidistrict litigation as it is quickly progressing. With the leadership structure in place, now is the time to begin preparing your claim. Our team of Bard PowerPort lawyers can guide you through the personal injury claims process to help you secure a fair resolution. Call 866-535-9515 or complete our simple online form for a free consultation with one of our knowledgeable personal injury lawyers today.
Choose Dolman Law Group to Handle Your Bard PowerPort Lawsuit
If you choose Dolman Law Group to handle your Bard PowerPort lawsuit, you will work with an experienced, dedicated legal team whose only mission is to represent you.
Dolman Law Group has a solid reputation for success in complex product liability cases, particularly those involving medical devices, defective drugs, and other serious product liability cases. Our attorneys possess a deep understanding of the intricacies involved in these lawsuits, and we’ll ensure your case is handled with the utmost professionalism and attention to detail.
With our proven track record of securing favorable outcomes for our clients, you can rest easy knowing we are committed to achieving justice and fair compensation for you. When you work with Dolman Law Group, you receive personalized care, transparent communication, and a relentless pursuit of your legal rights. Consider us an ideal choice for navigating the complexities of your Bard PowerPort lawsuit.
To learn more, call our compassionate team of legal professionals at (833) 606-DRUG [3784] or fill out our online contact form for a free consultation today.

